Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help with all parts of an inorganic pre-lab. Will attach an image. thank you so much! My assigned Diamine is ethylenediamine My assigned aldehyde
Need help with all parts of an inorganic pre-lab. Will attach an image. thank you so much! My assigned Diamine is ethylenediamine My assigned aldehyde is salicylaldehyde
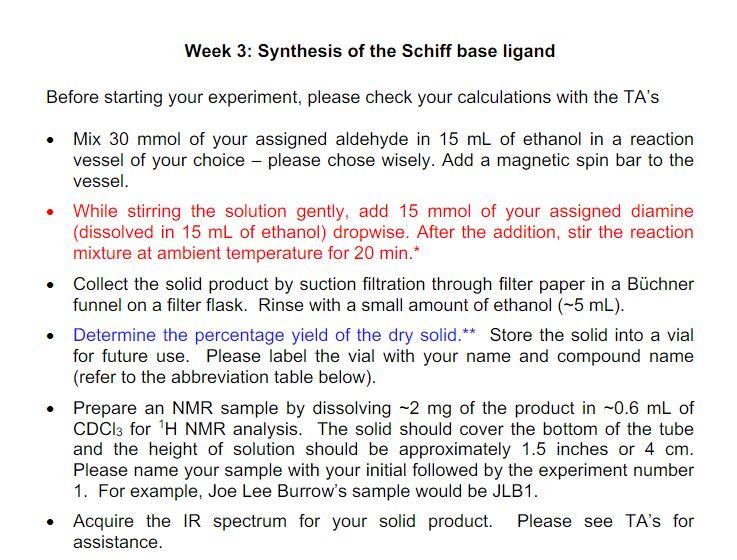
Will need 30 mmol of salicylaldehyde, and it will be mixed with 15 mL ethanol
Will need 15 mmol of ethylenediamine that will be added into mixture afterwards dropwise.
I believe the Schiff base abbreviation is H2 Salen
I need to know the formula weights for the aldehyde and diamine, and the quantity needed.
I will attach the lab procedure as well in case that helps.

what do you need to know? i will provide the structures of the aldehyde and diamine if that helps. whatever you can do is helpful thanks. 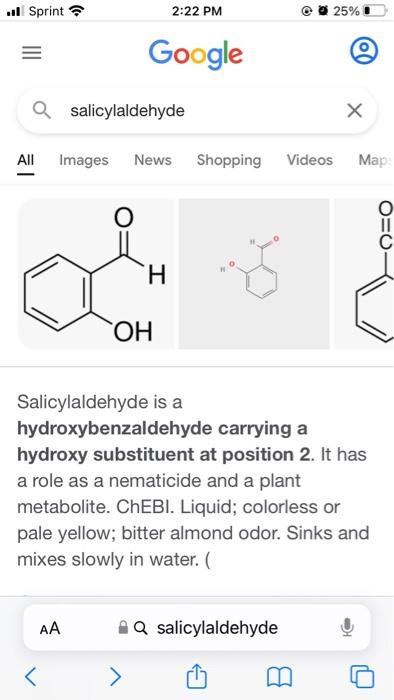
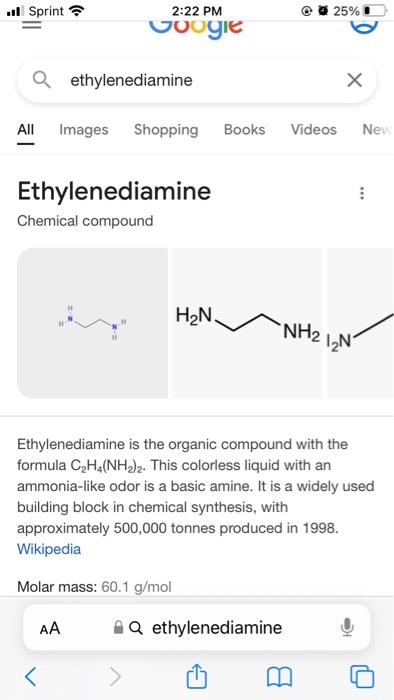
Week 3 Pre-lab Calculate the formula weights of the aldehyde (2 points) and diamine (2 points) that were assigned to you as well as the formula weight of the Schiff base ligand that you will make (2 points). Round the numbers to the nearest integers. If you get a half, round it up (for example, 23.5 will be 24). Calculate the quantities (solids in grams and liquids in milliliters) of the aldehyde (2 points) and diamine (2 points) that you will use for the experiments. Keep two significant numbers after the decimal point (for example, 1.20 grams or 2.23 mL). Which reagent(s) that you will use should be disposed as a halogenated material when not needed (2 points) Which reagent(s) that you will use are flammable liquids (2 points)? Are there any reagent(s) that you will use potential carcinogen(s) or suspected of causing cancer (1 point)? Week 3: Synthesis of the Schiff base ligand Before starting your experiment, please check your calculations with the TA's . Mix 30 mmol of your assigned aldehyde in 15 mL of ethanol in a reaction vessel of your choice - please chose wisely. Add a magnetic spin bar to the vessel. While stirring the solution gently, add 15 mmol of your assigned diamine (dissolved in 15 mL of ethanol) dropwise. After the addition, stir the reaction mixture at ambient temperature for 20 min.* Collect the solid product by suction filtration through filter paper in a Bchner funnel on a filter flask. Rinse with a small amount of ethanol (-5 mL). Determine the percentage yield of the dry solid.** Store the solid into a vial for future use. Please label the vial with your name and compound name (refer to the abbreviation table below). Prepare an NMR sample by dissolving -2 mg of the product in -0.6 mL of CDCl3 for 'H NMR analysis. The solid should cover the bottom of the tube and the height of solution should be approximately 1.5 inches or 4 cm. Please name your sample with your initial followed by the experiment number 1. For example, Joe Lee Burrow's sample would be JLB1. Acquire the IR spectrum for your solid product. Please see TA's for assistance. . ..ll Sprint 2:22 PM 25% Google a salicylaldehyde All Images News Shopping Videos : O ooo H OH Salicylaldehyde is a hydroxybenzaldehyde carrying a hydroxy substituent at position 2. It has a role as a nematicide and a plant metabolite. ChEBI. Liquid; colorless or pale yellow; bitter almond odor. Sinks and mixes slowly in water. Q salicylaldehyde ..ll Sprint 25% 2:22 PM voogie a ethylenediamine All Images Shopping Books Videos Ney Ethylenediamine Chemical compound H2N -NH2 1N Ethylenediamine is the organic compound with the formula C Ha(NH)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Wikipedia Molar mass: 60.1 g/mol AA AQ ethylenediamine 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


