Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help with both thanks If the actual isolated yield of pure isopropyl 3-(dimethylamino) benzoate is 12.54 g, calculate the % yield of the product.
Need help with both thanks 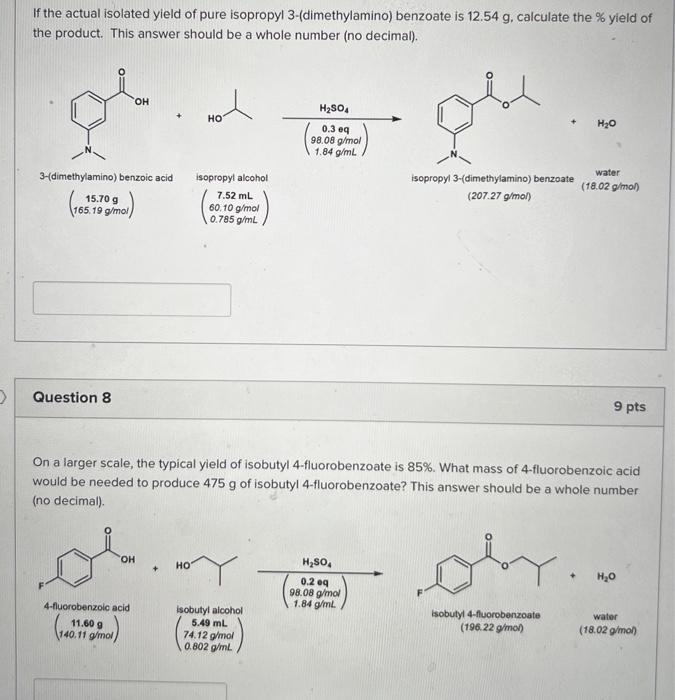
If the actual isolated yield of pure isopropyl 3-(dimethylamino) benzoate is 12.54 g, calculate the % yield of the product. This answer should be a whole number (no decimal). OH H2SO4 HO H2O 0.3 mg 98.08 g/mol 1.84 g/mL 3-(dimethylamino) benzoic acid 15.70 g 165.19 g/mol water (18.02 g/mol) isopropyl alcohol 7.52 mL 60.10 g/mol 0.785 g/mL isopropyl 3-(dimethylamino) benzoate (207.27 g/mol) Question 8 9 pts On a larger scale, the typical yield of isobutyl 4-fluorobenzoate is 85%. What mass of 4-fluorobenzoic acid would be needed to produce 475 g of isobutyl 4-fluorobenzoate? This answer should be a whole number (no decimal). OH H2SO4 HO + oby + H2O 0.2 09 98,08 g/mol 1.84 g/ml 4-fluorobenzoic acid 11.60 9 140.11 g/mol Isobutyl alcohol 5.49 mL 74.12 g/mol 0.802 g/ml isobutyl 4-fluorobenzoate (196.22 g/mol) water (18.02 g/mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


