Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with number 3 0. Calculate the volume of 1.0 M H2SO4 stock solution needed to prepare 150 mL of 0.20 M H2SO4 solution
need help with number 3 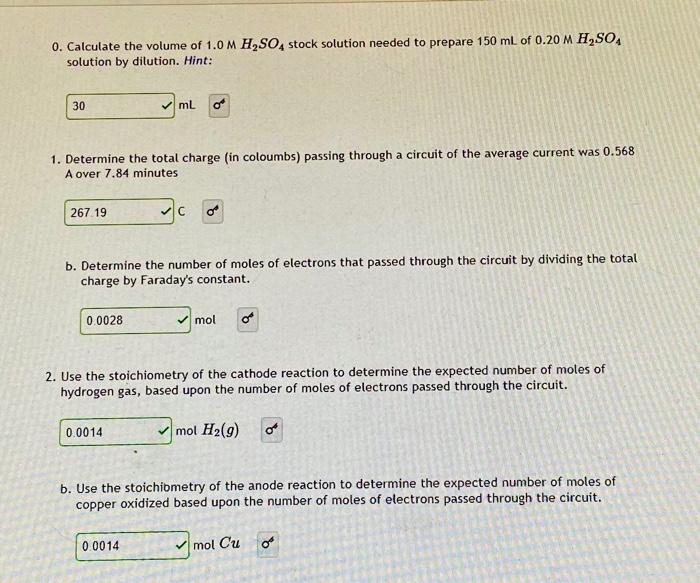
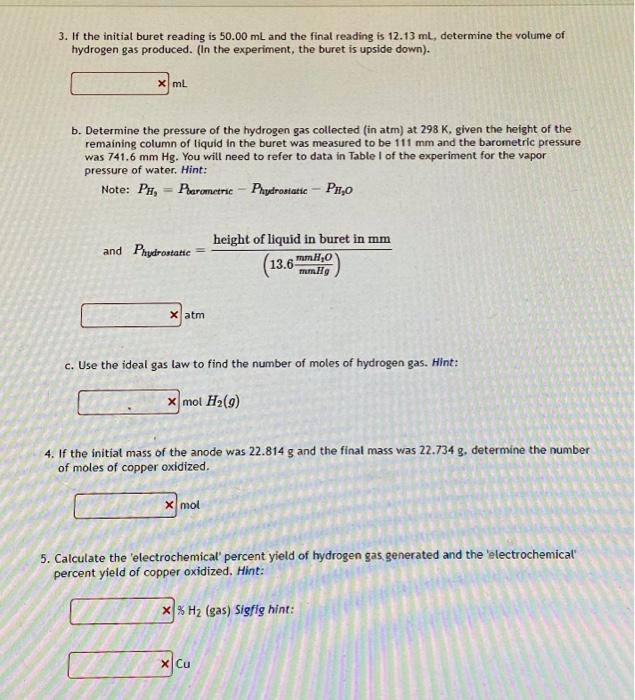
0. Calculate the volume of 1.0 M H2SO4 stock solution needed to prepare 150 mL of 0.20 M H2SO4 solution by dilution. Hint: 30 mL 1. Determine the total charge (in coloumbs) passing through a circuit of the average current was 0.568 A over 7.84 minutes 267.19 C b. Determine the number of moles of electrons that passed through the circuit by dividing the total charge by Faraday's constant. 0.0028 mol 2. Use the stoichiometry of the cathode reaction to determine the expected number of moles of hydrogen gas, based upon the number of moles of electrons passed through the circuit. 0.0014 mol H2(9) o b. Use the stoichiometry of the anode reaction to determine the expected number of moles of copper oxidized based upon the number of moles of electrons passed through the circuit. 0.0014 mol Cu Om 3. If the initial buret reading is 50.00 mL and the final reading is 12.13 ml, determine the volume of hydrogen gas produced. (In the experiment, the buret is upside down). xml b. Determine the pressure of the hydrogen gas collected (in atm) at 298 K. given the height of the remaining column of liquid in the buret was measured to be 111 mm and the barometric pressure was 741.6 mm Hg. You will need to refer to data in Table I of the experiment for the vapor pressure of water. Hint: Note: PH, = Parametric - Phydrostatic - PHO height of liquid in buret in mm and Phydrostatic 13.6m8,0 mmHg xatm c. Use the ideal gas law to find the number of moles of hydrogen gas. Hint: x mol H2(9) 4. If the initial mass of the anode was 22.814 g and the final mass was 22.734 g, determine the number of moles of copper oxidized. x mol 5. Calculate the electrochemical' percent yield of hydrogen gas generated and the electrochemical percent yield of copper oxidized. Hint: x%Hz (gas) Sigfig hint: x Cu 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


