Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help with questions 1 and 2 for my Chem lab Thanks TRIS HCl/ TRIS pH =7.20 pH=7.207.20=8.08+log(0.1Vb0.1Va)0.132=0.1Vbb/0.1VaVa+0.132=100mlVa=88.34mlVb=11.66ml 0.1 M Tris.Hcl: 88.34mL 0.1m Tris: 11.66mL
Need help with questions 1 and 2 for my Chem lab
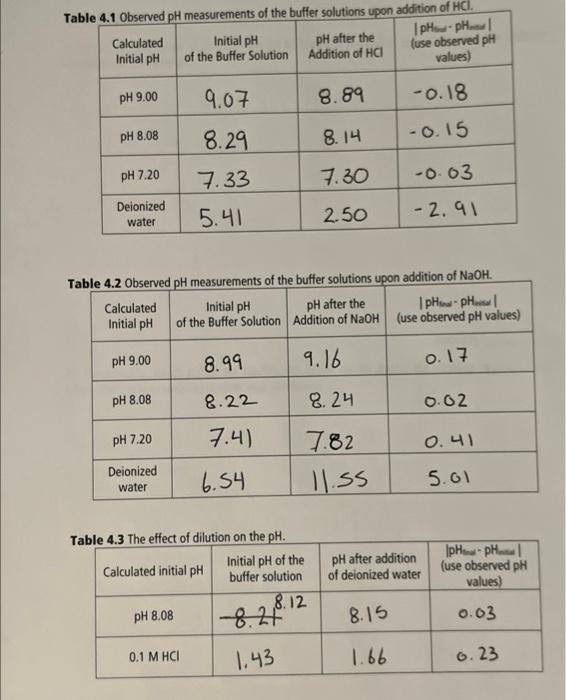
TRIS HCl/ TRIS pH =7.20 pH=7.207.20=8.08+log(0.1Vb0.1Va)0.132=0.1Vbb/0.1VaVa+0.132=100mlVa=88.34mlVb=11.66ml 0.1 M Tris.Hcl: 88.34mL 0.1m Tris: 11.66mL Tab T- h.1n a s nheanuad nH meacurements of the buffer solutions upon addition of NaOH. T 1) Calculate the final pH of 25mL of the 0.1M Tris HCl/ Tris buffer, pH=7.20, after the addition of 1.00mL of 0.1MNaOH. Final pH: 2) Theoretically, which pH for the Tris/Tris HCl buffer should display the smallest change in pH ? Why? Discuss the results to support your conclusion Thanks 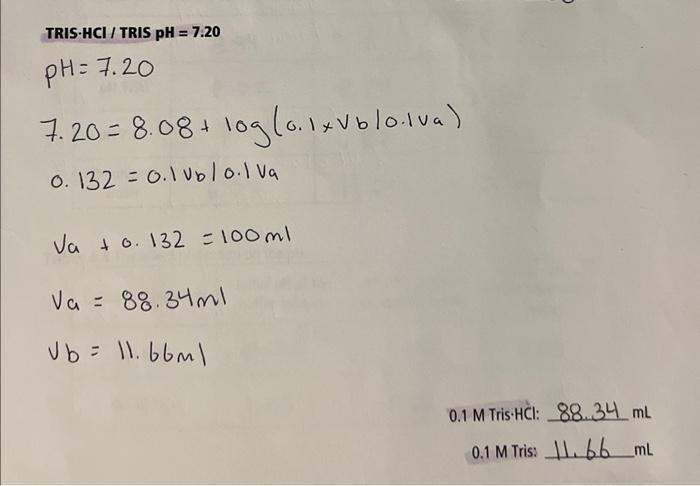

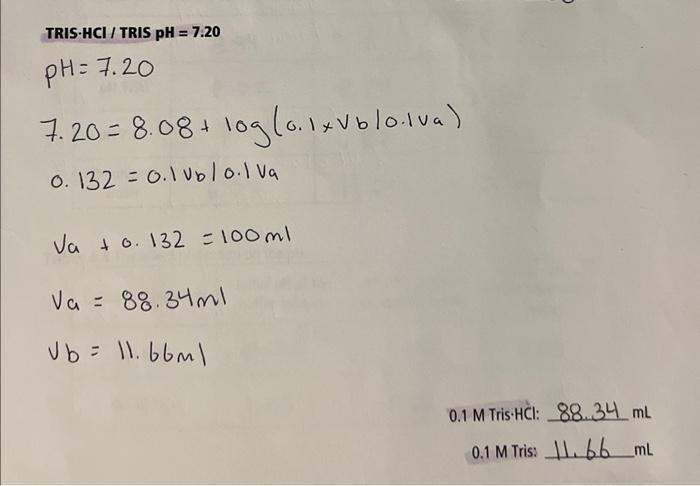


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


