Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with questions 2 a-c and 3 .2+ 1. (a) Ni @ Ni fae = -0.25 V S reduction Fel 3+ = -0.77 V
need help with questions 2 a-c and 3 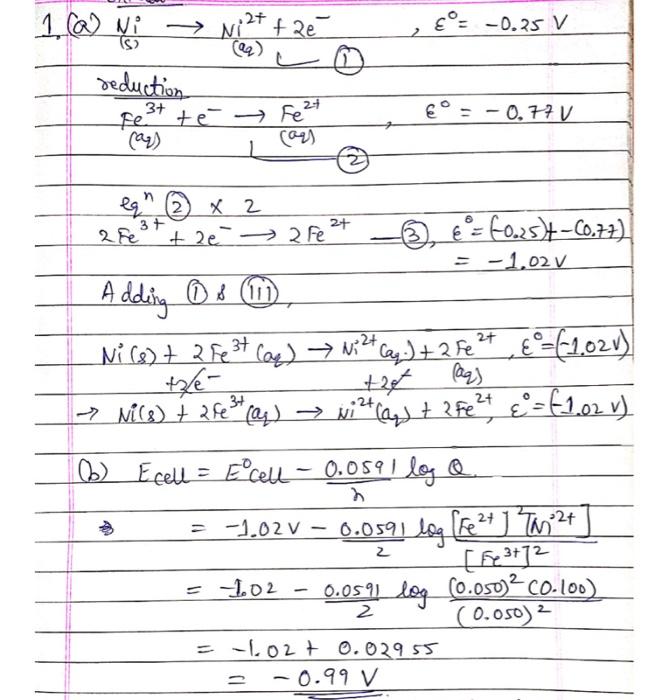

.2+ 1. (a) Ni @ Ni fae = -0.25 V S reduction Fel 3+ = -0.77 V te Fe 2+ 1 27 3+ .2+ 2+ egn x 2 3+ 2+ 2 Fe + 2e 2 Fe E = & = 60.25)+-60.77) = -1,02 v A dding Os (u Ni (s) + 2 Fe3+ a) Niztcaq.) + 2 fe2+, 6=(1,02v) (2 3+ aq + " E t- +2t as - Mis) + 3 (4) gita, + 3Fe + 4 = 1,02 v b) Ecell = Ecell - 0.0591 loge = = -1.02 V 0.0591 log [fe2+ ] Tw2+ ( [523112 = 102 0.05 y log co.oso)2C0.100) (0.050) 2 = 102t 0.02955 = -0.99 V $ 2 - 2 2. What steps would you take to solve each of the following common problems when building an electrochemical cell: a. The digital multimeter reads "O no matter what the setting on the dial. b. The reading "flickers" or fluctuates wildly, preventing an accurate reading, c. The calculated voltage is 0.0657V but the meter reads around 0.066V instead of the more accurate reading. d. You are getting a negative voltage instead of a positive voltage. 3. Use the equations for the cathode and the anode of the electrolytic cell that we are observing Hoffman cell). Explain why it takes one electron to produce a H+ at the anode and an OH at the cathode at the same time 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


