Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with table 2 1. Prepare the 4 half-cells as described in the handout. Two at a time, connect them with the salt bridge
need help with table 2 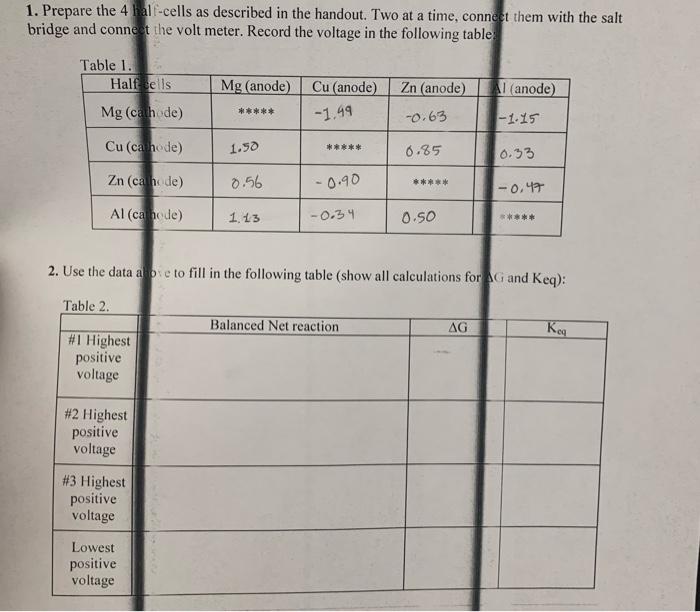
1. Prepare the 4 half-cells as described in the handout. Two at a time, connect them with the salt bridge and connect the volt meter. Record the voltage in the following table Table 1. Half cells Mg (anode) Cu (anode) Zn (anode) Al (anode) Mg (cathode) ***** -1.99 -0.63 -1.15 Cu(ca hode) 1.50 ***** 0.85 0.33 Zn (ca hede) 0.56 -0.90 ***** 1-0.47 Al (ca nede) 1.13 -0.34 0.50 *** 2. Use the data atoe to fill in the following table (show all calculations for AG and keq): Table 2. Balanced Net reaction AG Keg #1 Highest positive voltage #2 Highest positive voltage #3 Highest positive voltage Lowest positive voltage 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


