Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help with the graphing of this problem 2. Sulfur dioxide (SO2) is a gas pollutant that is released during combustion of coal. If the
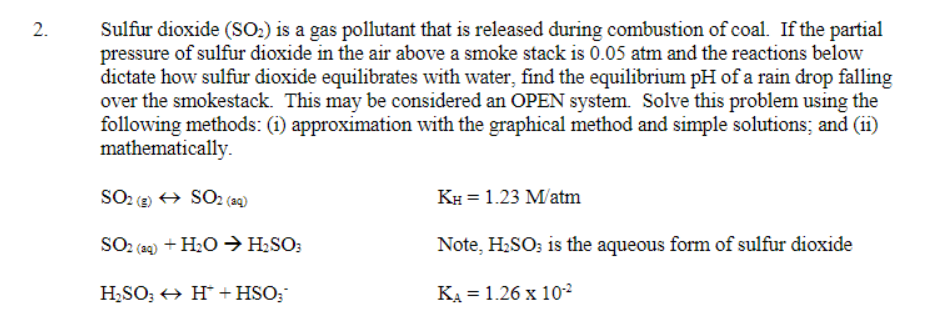
Need help with the graphing of this problem
2. Sulfur dioxide (SO2) is a gas pollutant that is released during combustion of coal. If the partial pressure of sulfur dioxide in the air above a smoke stack is 0.05 atm and the reactions below dictate how sulfur dioxide equilibrates with water, find the equilibrium pH of a rain drop falling over the smokestack. This may be considered an OPEN system. Solve this problem using the following methods: (1) approximation with the graphical method and simple solutions; and (11) mathematically. SO SO2 (aq) KH = 1.23 Matm SO2 (aq) + H2O + H2SO: Note, H2SO3 is the aqueous form of sulfur dioxide H2SO; HH* + HSO: KA = 1.26 x 102Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


