Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need Need help with the graphing part especially Enter the mass values for each trial in the table below. Calculate the average mass value and
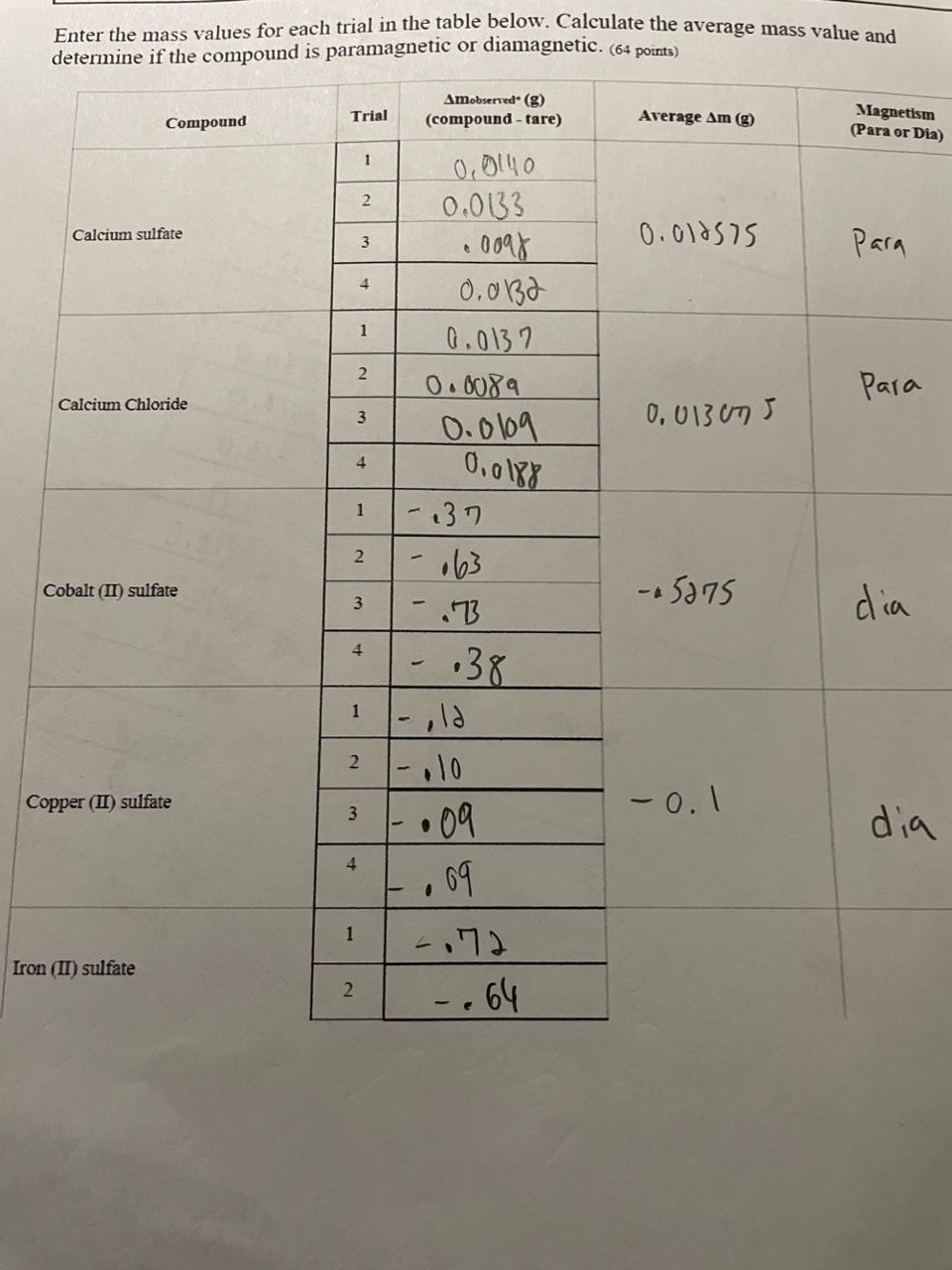 need
need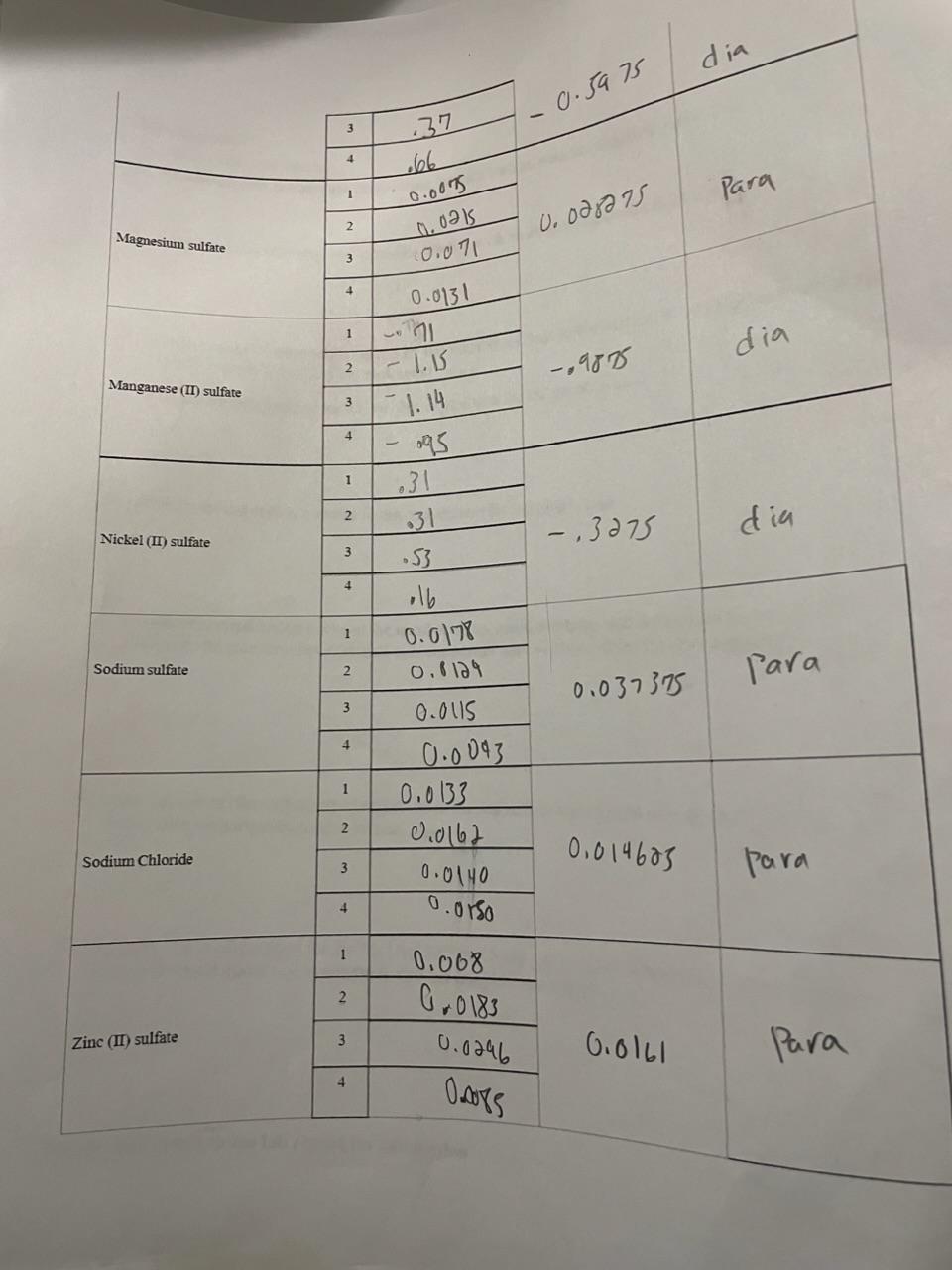

Need help with the graphing part especially
Enter the mass values for each trial in the table below. Calculate the average mass value and Discussion 1. Summarize your data by ... a. describing how paramagnetic materials interact with an external magnetic field. (2pts) b. describing how diamagnetic materials interact with an external magnetic field. (2ps) 2. Create a computer-generated graph of the number of unpaired electrons in a compound versus the average change in mass of the compound. (16 pts) 3. Identify the independent variable in this experiment for the graph. (2pts) 4. Identify the dependent variable in this experiment for the graph. (2pts) 5. Explain the relationship between the number of unpaired electrons and the change in mass that was observed. Be sure that this explanation is supported with evidence from the data collected. (4 pts) 6. Does the anion (the negatively charged ion in the salt) affect the change is mass observed? Support your claim with experimental evidence. (4 pts) 7. The electron configuration for Zn2+ is [Ar]3d10 and NOT [Ar]4s23d8. What experimental evidence collected would allow one to conclude that the [Ar]3d10 is correct? (4 pts) Attach your graph to the lab report for submissionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


