Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need questions 3, 4,5,6 . Results and Calculations 1. Write balanced equations for the synthesis of the two complexes. 2. Describe physical appearance of the
need questions 3, 4,5,6 . Results and Calculations 1. Write balanced equations for the synthesis of the two complexes. 2. Describe physical appearance of the two complexes. 3. Why does cis-complex precipitates first? Explain why is it possible to convert cis isomer to trans by heating. 4. Calculate % yield of the cis-Cu(glyo'),.H,O complex. 5. In addition to the IR spectra that you obtained for the two isomers, you are provided with the two far infrared spectra (Fig 3). Use all four IR spectra to assign appropriate IR vibrations for the following group frequencies: v,(COO); Vas(COO), v, (NH), v,(CH), v (CH). v,(OH). v, (CUN), vs (CuN). v,(CuO), Vas (CuO). What are the major similarities and major differences in the IR spectra for the two isomers? 6. Can you suggest why IR spectrum of the trans isomer is much simpler than that of the cis. 
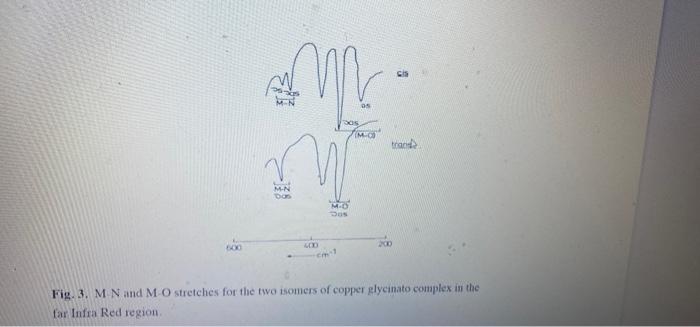


Results and Calculations 1 Write balanced equations for the synthesis of the two complexes. 2. Describe physical appearance of the two complexes. 3. Why does cis complex precipitates first? Explain why is it possible to convert cis isomer to trans by heating. 4 Calculate yield of the cis-Cu(glyo), H.O complex 5 In addition to the IR spectra that you obtained for the two isomers, you are provided with the two far infrared spectra (Fig 3). Use all four IR spectra to assign appropriate IR vibrations for the following group frequencies: u.(COO); w..(COO)., (NH). (CH). U. (CH), COH), (CUN). (CN) 2.(Cu)... (Cho) What are the major similarities and major differences in the IR spectra for the two isomers 6 Can you suggest why IR spectrum of the trams isomer is unch simpler than that of the m cis OS MO trand M-N DO MO Dos Fig. 3. M N and M O stretches for the two isomers of copper glycinato complex in the far Infra Red region Synthesis of cis-Culglyo),.H.0 Transfer 100 mL of distilled water into 150 ml beaker and heat on a hotplate to 70C. Use a thermometer to adjust the hotplate setting to 70C. To a 50 ml. Erlenmeyer flask add 20 ml of ethanol and heat to 70 C. (Warning: DO NOT HEAT ETHANOL ABOVE 70C-IT IS FLAMABLE!!!) Weigh 10 g 0.01 mole) of Copper(II) acetate monohydrate into 100 ml beaker Add 20 ml of hot water and stir until copper acetate is completely dissolved. Add 20 ml of hot absolute ethanol, stir and keep hot on the hotplate. Weigh 0.75 g (0.02 mole) of Glycine into 100 ml. beaker and dissolve in 20 mL of hot water. Add hot Glycine solution to the hot copper acetate solution and stir well using stirrinie rod. Cool copper acetate lycme mixture in the ice bath and allow needle like crystals of ciscocelo), Ho te precipitate This will take about 10-15 minutes Suction filter the crystals, wash with cold ethanol and air dry. Transfer dried crystals to the previously weighed weithing bottle and obtain the mass of the synthesized crystal Record the color and the appearance of the synthesized complex Obtain the IR spects of the complex Sare-02s of the complex crystals for conversion to raisomer all equorum mixture of the two isomers: 2 Cu(CH,CO2, H2O + 4 H2NCH,CO.H>cis- Cu(gly ,HO + trans Cu(gly ,H,O + 2 CHCO,H (1) The cis-isomer precipitates much more quickly than the -trans. This leads to a shift in the equilibrium away from the more thermodynamically stable trans isomer, giving only the more kinetically favored els -isomer. The cis-isomer can be converted to the trans by heating at 180C for 15 min. Experimental Procedure: Reagents. Copper (11) acetate monohydrate Cu(CH.CO.), H.O(MW 199.65 g/mol): Glycine (MW 7507 g/mol). absolute ethanol, distilled water: Equipment 2x100 ml beakers, 50 ml. Erlenmiyet Flask, Graduated cylinder, glass stirring rods, weighing bottle, hot plate and thermometer - stiction filtration apparatus buehner funnel and 500 mL side arm filtering flask Ice bath 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


