Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need solution for question 3 2. The molar enthalpy of a binary solution is given by h=300x1+1000x2+(5x1+10x2)x1x2 Obtain the numerical value of eufe-omponent enthatpies, partial
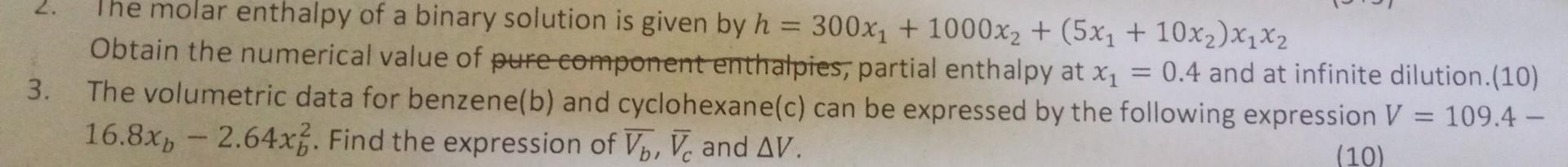

Need solution for question 3
2. The molar enthalpy of a binary solution is given by h=300x1+1000x2+(5x1+10x2)x1x2 Obtain the numerical value of eufe-omponent enthatpies, partial enthalpy at x1=0.4 and at infinite dilution.(10) 3. The volumetric data for benzene(b) and cyclohexane(c) can be expressed by the following expression V=109.4 16.8xb2.64xb2. Find the expression of Vb,Vc and V (10) 3. The volumetric data for benzene(b) and cyclohexane(c) can be expressed by the following expression V=109.4 16.8xb2.64xb2. Find the expression of Vb,Vc and V. 4. (a) The excess Gibbs free energy of a binary liquid system can be expressed as RTGE=0.5x1x2. Obtain the activityStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


