Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need to be solved in MATLAB This question ne Q2. The dissolution of copper sulfide in aqueous nitric acid is described by the following chemical
Need to be solved in MATLAB
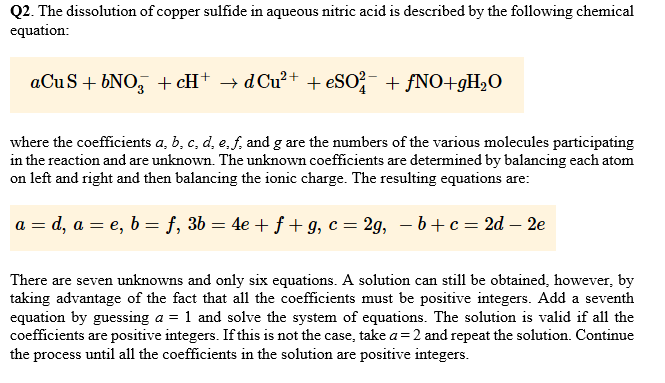
This question ne
Q2. The dissolution of copper sulfide in aqueous nitric acid is described by the following chemical equation: where the coefficients a, b, c, d, e,f, and g are the numbers of the various molecules participating in the reaction and are unknown. The unknown coefficients are determined by balancing each atom on left and right and then balancing the ionic charge. The resulting equations are: There are seven unknowns and only six equations. A solution can still be obtained, however, by taking advantage of the fact that all the coefficients must be positive integers. Add a seventh equation by guessing a -1 and solve the system of equations. The solution is valid if all the coefficients are positive integers. If this is not the case, take a 2 and repeat the solution. Continue the process until all the coefficients in the solution are positive integersStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


