Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Needed Information Molar mass of Ba(OH)2 = 171.35 g/mol If 50.0 grams of Ba(OH), is dissolved in 750 g calculate %(m/m) Report answer to
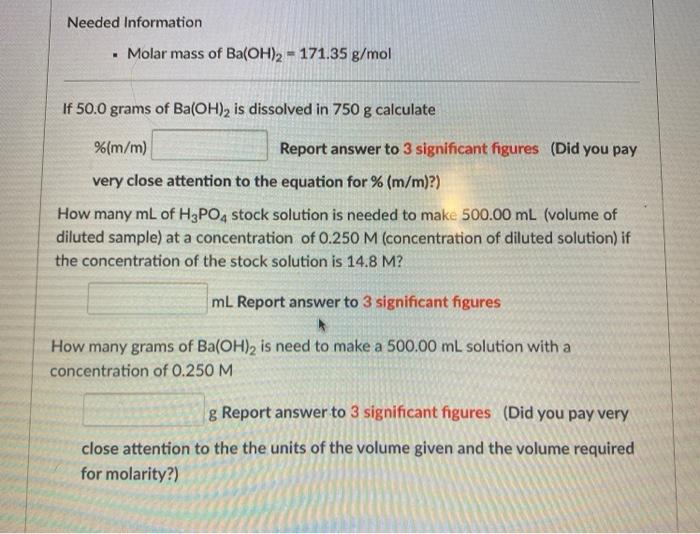
Needed Information Molar mass of Ba(OH)2 = 171.35 g/mol If 50.0 grams of Ba(OH), is dissolved in 750 g calculate %(m/m) Report answer to 3 significant figures (Did you pay very close attention to the equation for % (m/m)?) How many mL of H3PO, stock solution is needed to make 500.00 mL (volume of diluted sample) at a concentration of 0.250 M (concentration of diluted solution) if the concentration of the stock solution is 14.8 M? mL Report answer to 3 significant figures How many grams of Ba(OH)2 is need to make a 500.00 mL solution with a concentration of 0.250 M g Report answer to 3 significant figures (Did you pay very close attention to the the units of the volume given and the volume required for molarity?)
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Selutuon Seluto Solvent 50 750g ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


