Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Needing help with the plot and critical point of the problem 3. 0.1 gram of solid aluminum phosphate (Al(PO4) (s) is added to 500 L
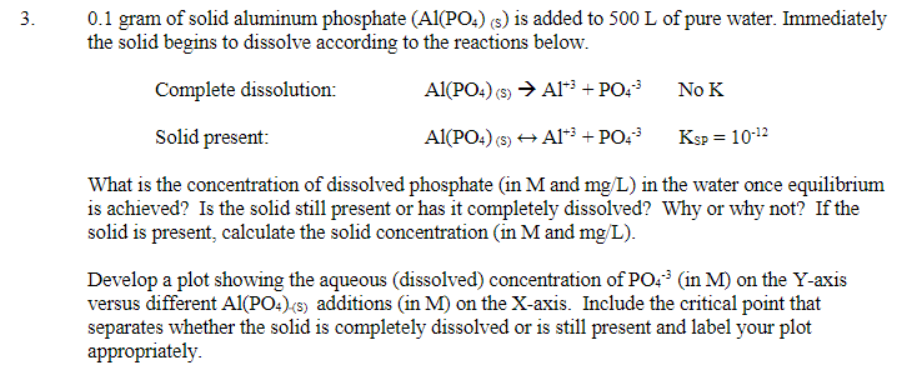
Needing help with the plot and critical point of the problem
3. 0.1 gram of solid aluminum phosphate (Al(PO4) (s) is added to 500 L of pure water. Immediately the solid begins to dissolve according to the reactions below. Complete dissolution: Al(PO4) () A1** + PO! NOK Solid present Al(PO4) (s) + A1? + PO43 Ksp = 10-12 What is the concentration of dissolved phosphate (in M and mg/L) in the water once equilibrium is achieved? Is the solid still present or has it completely dissolved? Why or why not? If the solid is present, calculate the solid concentration (in M and mg/L). Develop a plot showing the aqueous (dissolved) concentration of PO." (in M) on the Y-axis versus different Al(PO4)-(s) additions in M) on the X-axis. Include the critical point that separates whether the solid is completely dissolved or is still present and label your plot appropriatelyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


