Question
Ninety-five (95%) of acetone is to be removed from a gas air stream entering at 100 kmol/hr containing 85 mol% of air and 15 mol%
Ninety-five (95%) of acetone is to be removed from a gas air stream entering at 100 kmol/hr containing 85 mol% of air and 15 mol% of acetone by countercurrent contact with pure water in a sieve-tray column with expected overall tray efficiency if 50% (EO = 0.5). The tray hole diameter is 4.5 mm on an equilateral triangle of 12 mm pitch. The column will operate at 293 K and 101 kPa. Equilibrium data for acetone-water at these conditions are:
mol % of acetone in water 0 3.30 7.20 11.7 17.1 Acetone partial P in air, torr 0 30.00 62.80 85.40 103.0
Determine the following (for full credit, draw a well labeled diagram, state assumptions, and properly label axis in graphs):
a. b.
c. d. e.
f. g.
Given: Mair = 29 Macetone = swater = 70 dyne/cm rwater = 1 g/cm3 = 1000 kg/m3 Foaming factor = 1 Flooding factor = 0.7 Tray spacing = 0.5 1 kPa = 7.5 torr R = 8.134 kPa m3/kmol-K
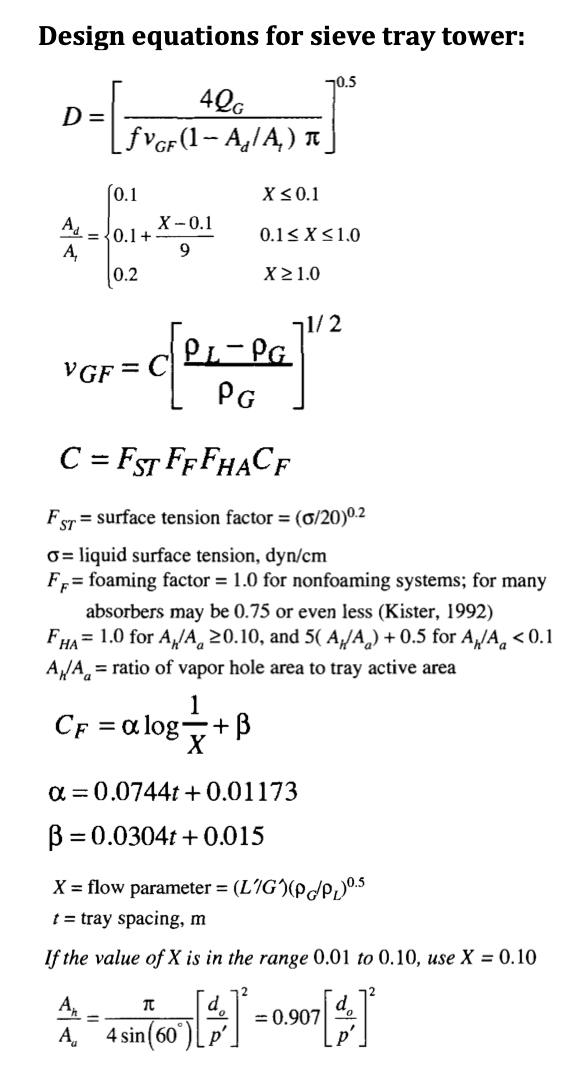
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


