Answered step by step
Verified Expert Solution
Question
1 Approved Answer
nit for sure what i did wrong in my equation Microwave ovens work by irradiating food with microwave radiation, which is absorbed and converted into
nit for sure what i did wrong in my equation 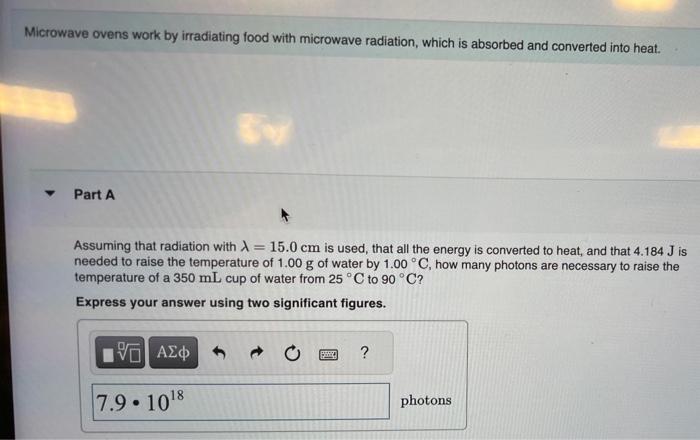
Microwave ovens work by irradiating food with microwave radiation, which is absorbed and converted into heat. Part A Assuming that radiation with =15.0cm is used, that all the energy is converted to heat, and that 4.184J is needed to raise the temperature of 1.00g of water by 1.00C, how many photons are necessary to raise the temperature of a 350mL cup of water from 25C to 90C ? Express your answer using two significant figures 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


