Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Nitric oxide emissions from diesel automobile exhaust can be reduced using a catalytic converter that is 100 mm long and consists of many channels
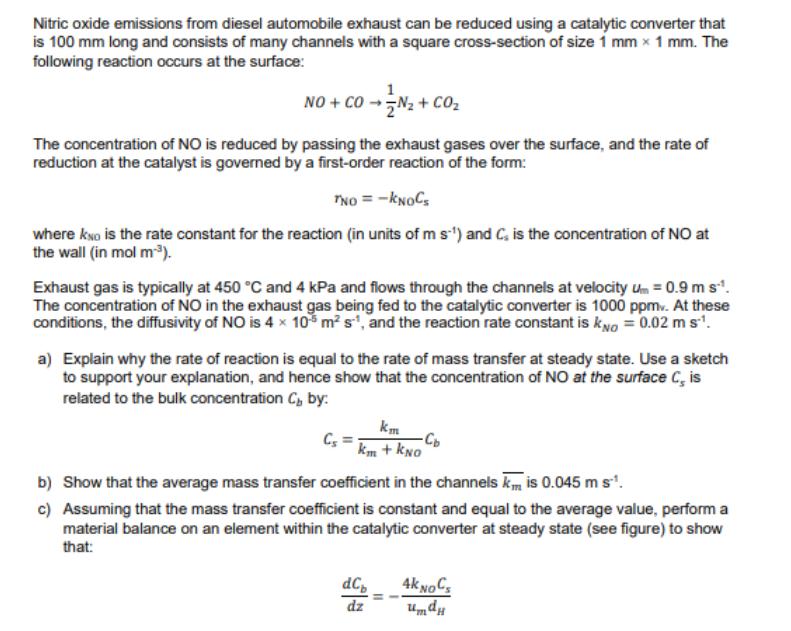

Nitric oxide emissions from diesel automobile exhaust can be reduced using a catalytic converter that is 100 mm long and consists of many channels with a square cross-section of size 1 mm 1 mm. The following reaction occurs at the surface: NO+CON+ CO The concentration of NO is reduced by passing the exhaust gases over the surface, and the rate of reduction at the catalyst is governed by a first-order reaction of the form: TNO = -KNOC where KNO is the rate constant for the reaction (in units of m s) and C, is the concentration of NO at the wall (in mol m). Exhaust gas is typically at 450 C and 4 kPa and flows through the channels at velocity um = 0.9 m s**. The concentration of NO in the exhaust gas being fed to the catalytic converter is 1000 ppm. At these conditions, the diffusivity of NO is 4 105 m s, and the reaction rate constant is kno = 0.02 m s. a) Explain why the rate of reaction is equal to the rate of mass transfer at steady state. Use a sketch to support your explanation, and hence show that the concentration of NO at the surface C, is related to the bulk concentration C, by: km km + KNO b) Show that the average mass transfer coefficient in the channels k, is 0.045 m s. c) Assuming that the mass transfer coefficient is constant and equal to the average value, perform a material balance on an element within the catalytic converter at steady state (see figure) to show that: dCp dz 4K NOC umdu d) Integrate the expression from (c), analytically or otherwise, and hence determine the removal efficiency, which is defined by: Cout eff=1-- Cin z+dz C+dCb Co Cout -KNOC
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


