Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Nitrogen and hydrogen react to form ammonia, like this: N2(g)+3H2(g)2NH3(g) Use this chemical equation to answer the questions in the table below. begin{tabular}{l|l} hline Suppose
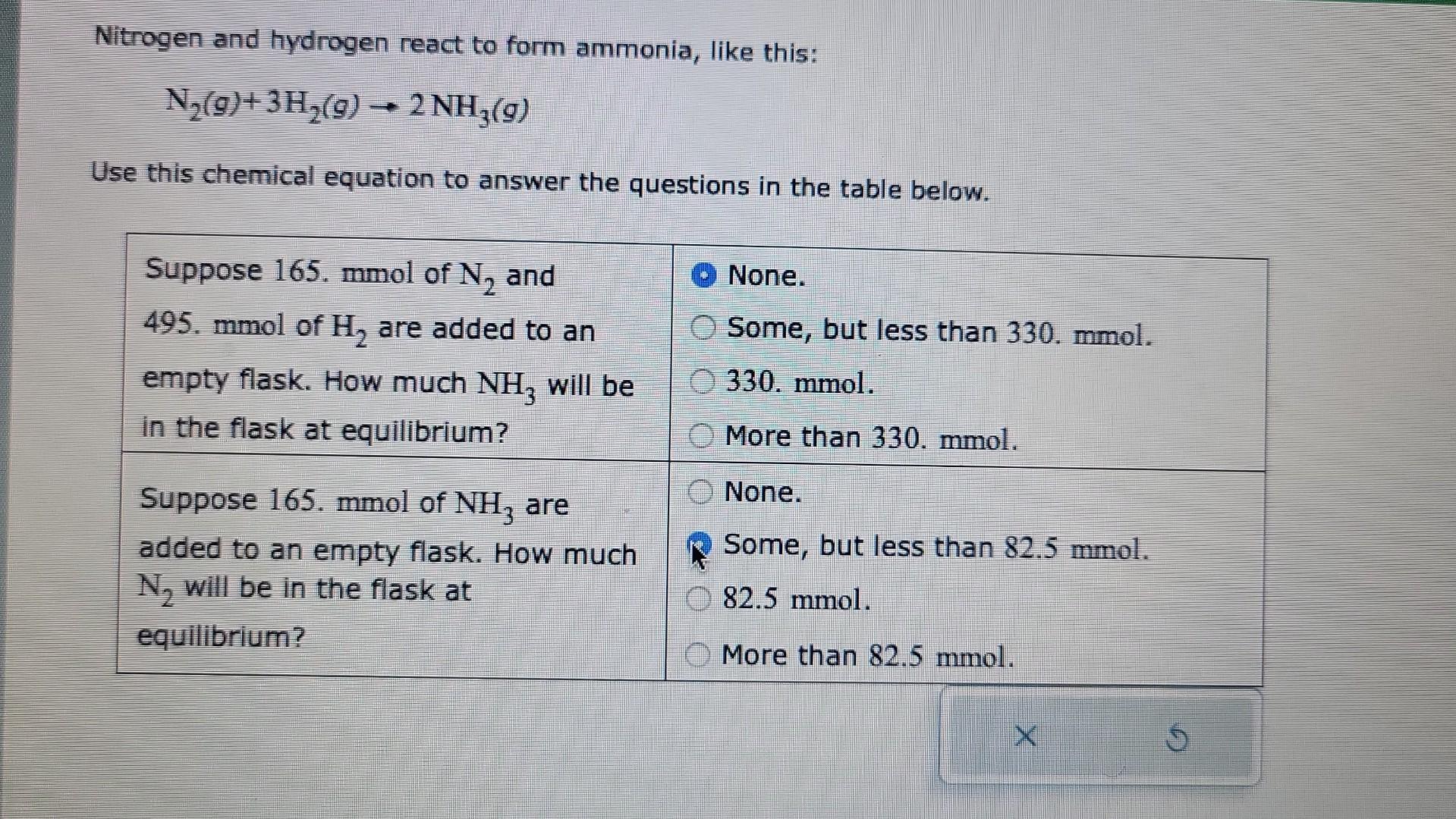
Nitrogen and hydrogen react to form ammonia, like this: N2(g)+3H2(g)2NH3(g) Use this chemical equation to answer the questions in the table below. \begin{tabular}{l|l} \hline Suppose 165. mmol of N2 and 495. mmol of H2 are added to an empty flask. How much NH3 will be in the flask at equilibrium? & None. \\ Some, but less than 330.mmol. \\ Suppose 165. mmol of NH3 are & More than 330.mmol. \\ added to an empty flask. How much \\ N2 will be in the flask at \\ equilibrium? \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


