Answered step by step
Verified Expert Solution
Question
1 Approved Answer
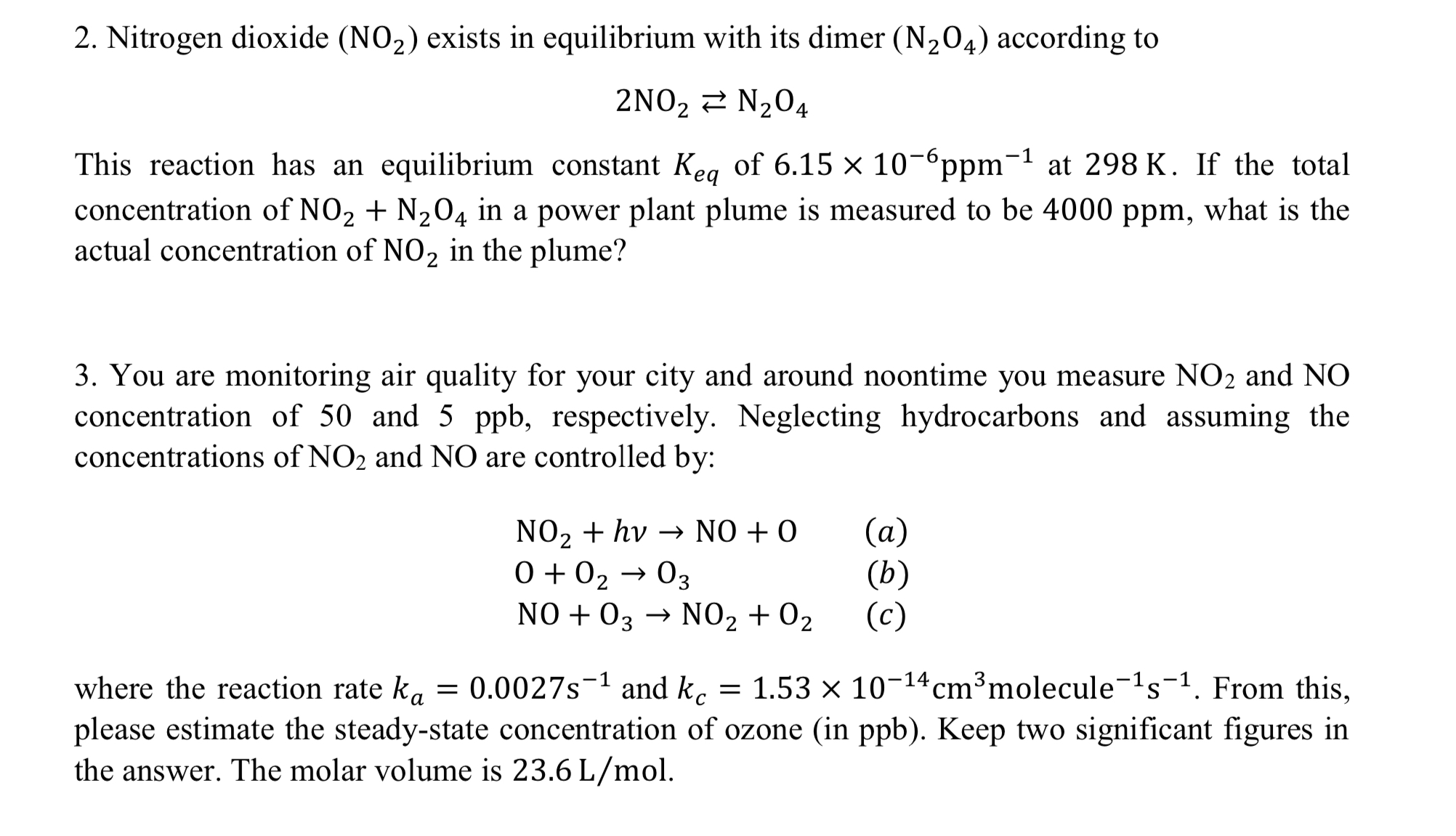
Nitrogen dioxide ( N O 2 ) exists in equilibrium with its dimer ( N 2 O 4 ) according to 2 N O 2
Nitrogen dioxide exists in equilibrium with its dimer according to
This reaction has an equilibrium constant of at If the total concentration of in a power plant plume is measured to be what is the actual concentration of in the plume?
You are monitoring air quality for your city and around noontime you measure and concentration of and respectively. Neglecting hydrocarbons and assuming the concentrations of and are controlled by:
where the reaction rate and From this, please estimate the steadystate concentration of ozone in ppb Keep two significant figures in the answer. The molar volume is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


