Question
Nitrogen gas is used in a Carnot cycle (see sketch, Processes 12 and 3-4 are isothermal; processes 23 and 41 are adiabatic). The cycle
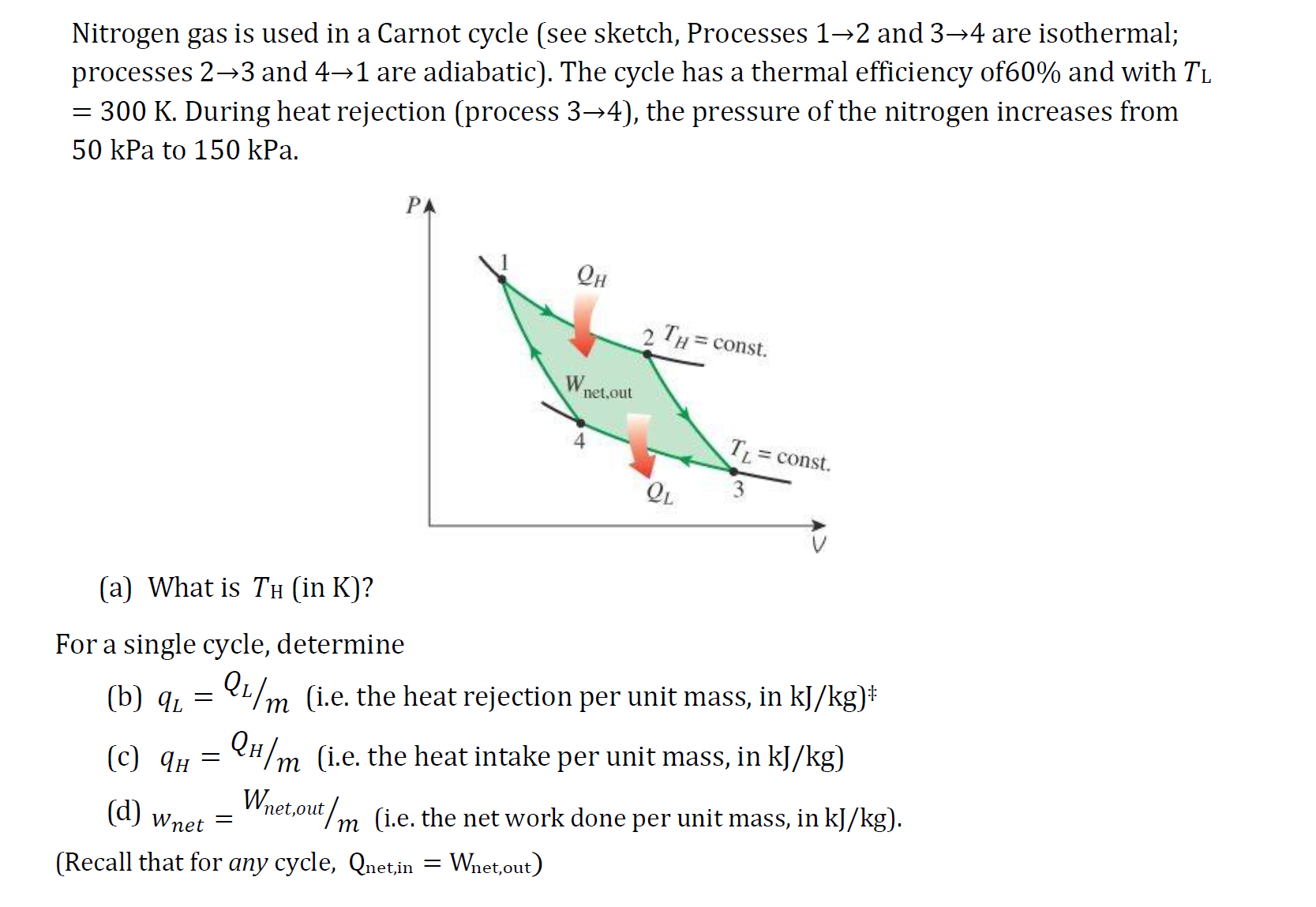
Nitrogen gas is used in a Carnot cycle (see sketch, Processes 12 and 3-4 are isothermal; processes 23 and 41 are adiabatic). The cycle has a thermal efficiency of60% and with TL = 300 K. During heat rejection (process 34), the pressure of the nitrogen increases from 50 kPa to 150 kPa. (a) What is TH (in K)? For a single cycle, determine (b) qL () ( - PA = QH (d) Wnet (Recall that for any cycle, Qnet,in = Wnet,out) Wnet.out 2 TH=const. TL QL 3 QL/m (i.e. the heat rejection per unit mass, in kJ/kg)* QH/m (i.e. the heat intake per unit mass, in kJ/kg) = const. Wnet,out/m (i.e. the net work done per unit mass, in kJ/kg).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Explanation Calculating the T H q H q L and W net for given carnot cycle Given data ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Thermodynamics
Authors: Richard E. Sonntag, Claus Borgnakke, Gordon J. Van Wylen
6th edition
471152323, 978-0471152323
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



