Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Nitrogen is bubbled through a liquid mixture that initially contains 40.0 mole% benzene and the remainder toluene. The system pressure is 2.5atm and the temperature
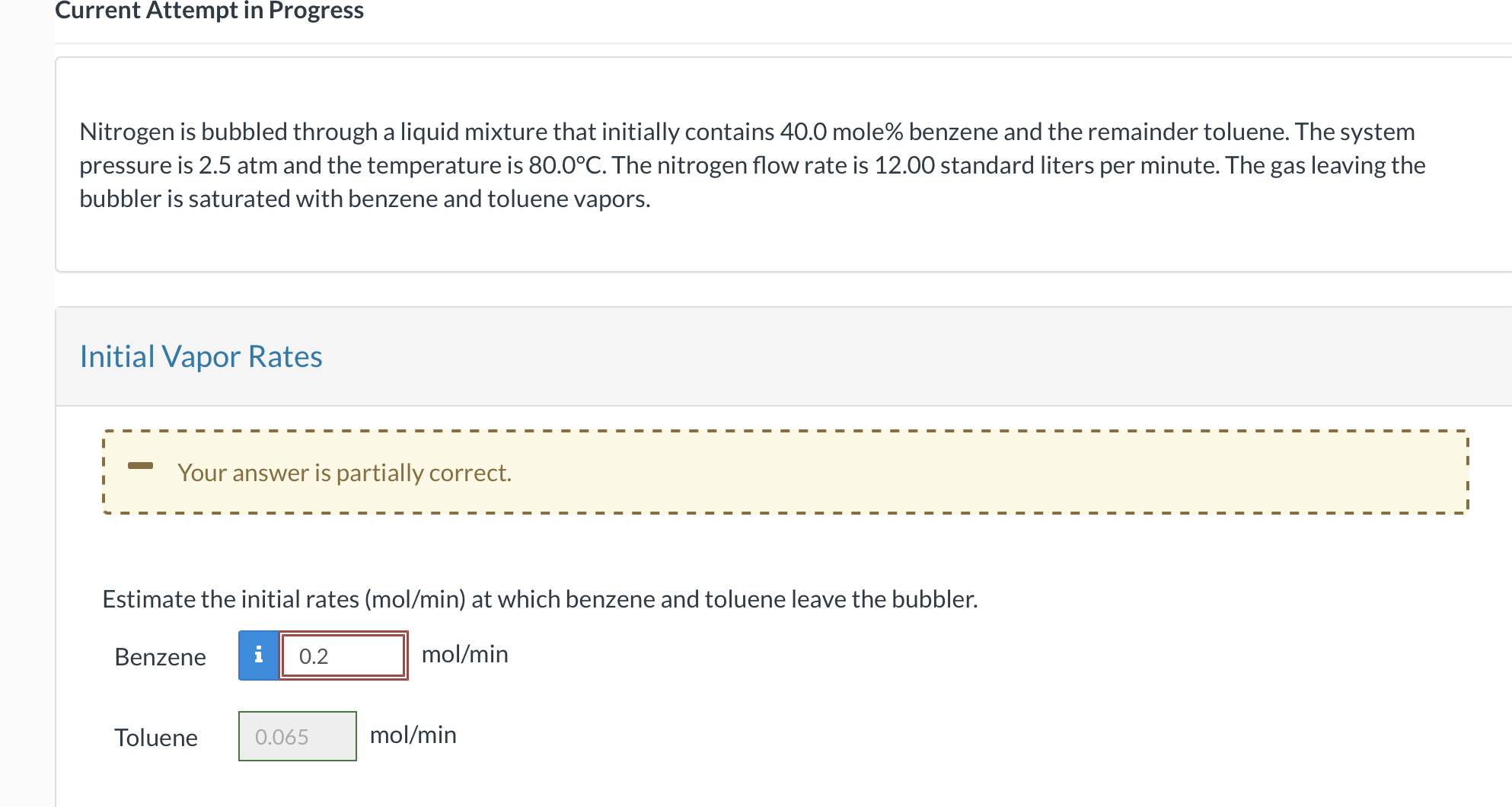 Nitrogen is bubbled through a liquid mixture that initially contains 40.0 mole\% benzene and the remainder toluene. The system pressure is 2.5atm and the temperature is 80.0C. The nitrogen flow rate is 12.00 standard liters per minute. The gas leaving the bubbler is saturated with benzene and toluene vapors. Initial Vapor Rates Estimate the initial rates ( mol/min ) at which benzene and toluene leave the bubbler. Benzene mol/min Toluene mol/min Nitrogen is bubbled through a liquid mixture that initially contains 40.0 mole\% benzene and the remainder toluene. The system pressure is 2.5atm and the temperature is 80.0C. The nitrogen flow rate is 12.00 standard liters per minute. The gas leaving the bubbler is saturated with benzene and toluene vapors. Initial Vapor Rates Estimate the initial rates ( mol/min ) at which benzene and toluene leave the bubbler. Benzene mol/min Toluene mol/min
Nitrogen is bubbled through a liquid mixture that initially contains 40.0 mole\% benzene and the remainder toluene. The system pressure is 2.5atm and the temperature is 80.0C. The nitrogen flow rate is 12.00 standard liters per minute. The gas leaving the bubbler is saturated with benzene and toluene vapors. Initial Vapor Rates Estimate the initial rates ( mol/min ) at which benzene and toluene leave the bubbler. Benzene mol/min Toluene mol/min Nitrogen is bubbled through a liquid mixture that initially contains 40.0 mole\% benzene and the remainder toluene. The system pressure is 2.5atm and the temperature is 80.0C. The nitrogen flow rate is 12.00 standard liters per minute. The gas leaving the bubbler is saturated with benzene and toluene vapors. Initial Vapor Rates Estimate the initial rates ( mol/min ) at which benzene and toluene leave the bubbler. Benzene mol/min Toluene mol/min Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


