Answered step by step
Verified Expert Solution
Question
1 Approved Answer
NON IDEAL carbon dioxide is adiabatically compressed to 7000kPa. It had initial pressure 101.325kPa and initial temperature of 303K. Using the equation of state P=(RT)/(v
NON IDEAL carbon dioxide is adiabatically compressed to 7000kPa. It had initial pressure 101.325kPa and initial temperature of 303K. Using the equation of state P=(RT)/(v - b) and the thermodynamic equation of state (in picture below), find the final temperature of the NON IDEAL carbon dioxide given that R = 8.3145J/mol/K and c_v = 0.03kJ/mol/K Mayers relation cannot be used as the CO2 is non ideal.
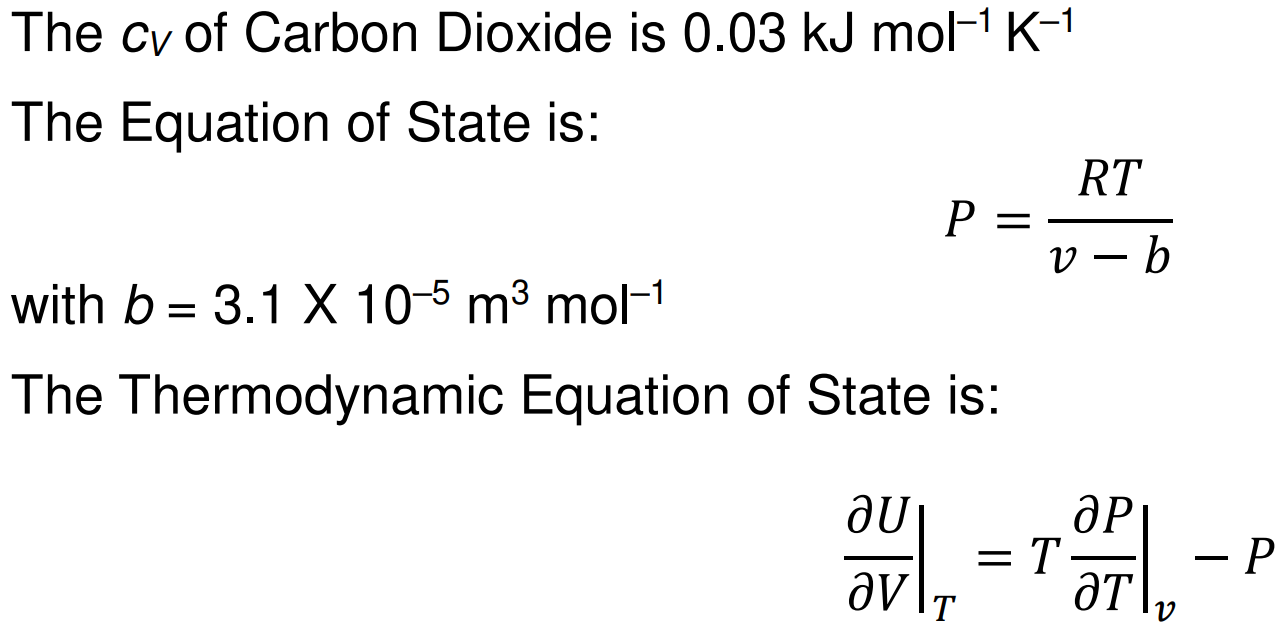 The cV of Carbon Dioxide is 0.03kJmol1K1 The Equation of State is: P=vbRT with b=3.1105m3mol1 The Thermodynamic Equation of State is: VUT=TTPvP
The cV of Carbon Dioxide is 0.03kJmol1K1 The Equation of State is: P=vbRT with b=3.1105m3mol1 The Thermodynamic Equation of State is: VUT=TTPvP Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


