Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Not sure how ti do the summary results mor the last two questions. These results are all based on my previous results from the tabels
Not sure how ti do the summary results mor the last two questions. These results are all based on my previous results from the tabels included in this right below. 

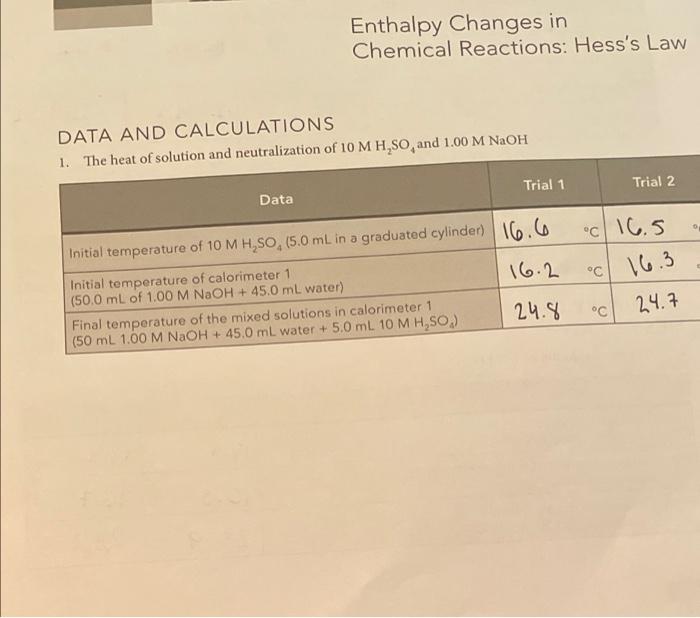
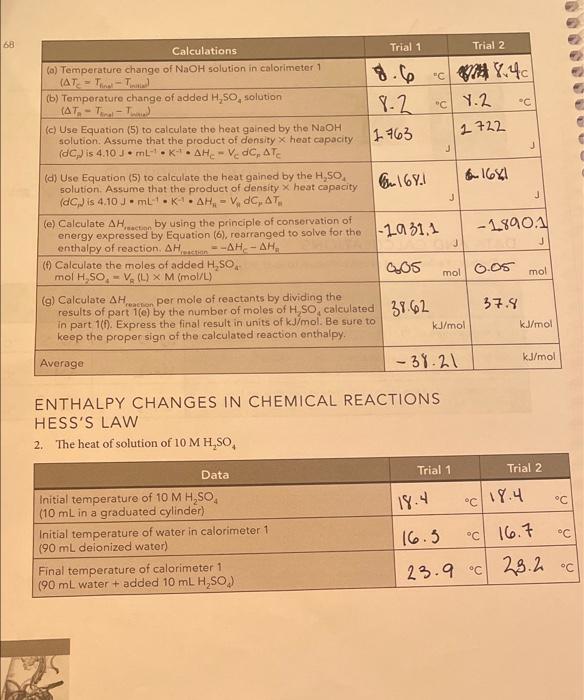
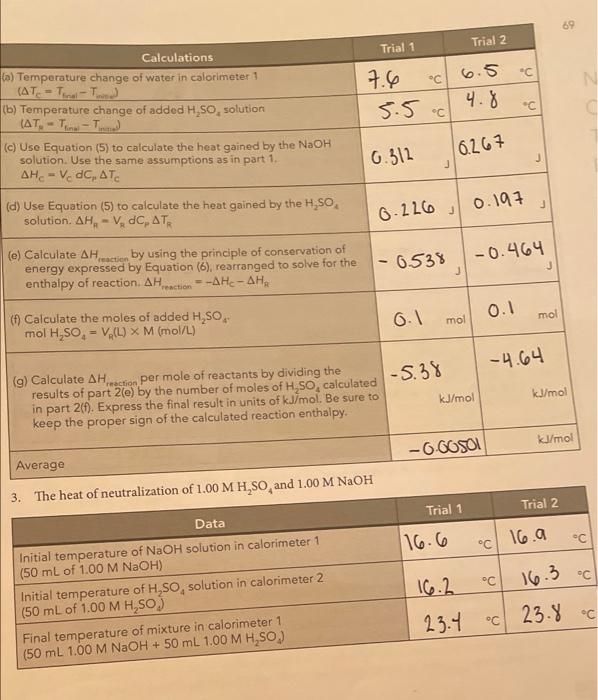
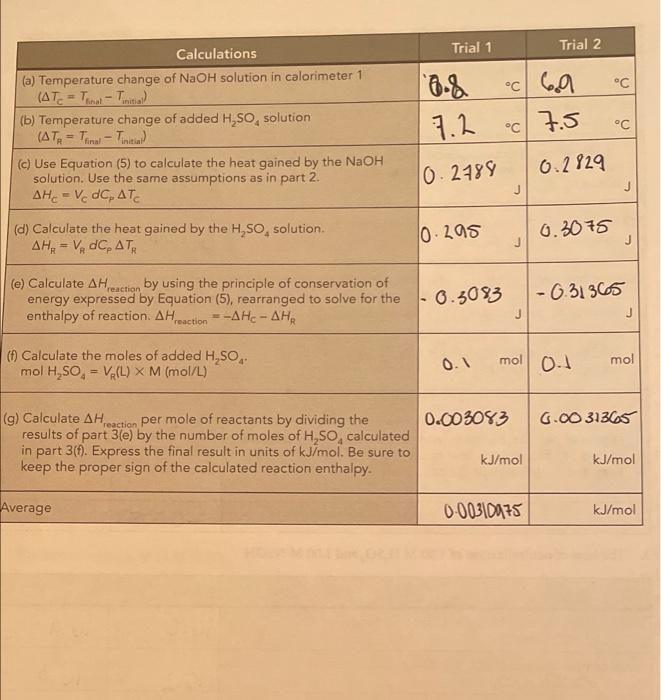
71 N SUMMARY OF RESULTS Write the chemical equations corresponding to the reactions of parts 1, 2, and 3, and summarize the calculated heats of reaction. AH..in units of kJ/mol. Chemical equations Average Auction kJ/mol) NaOH + H504N028 **0 31.21 Part 2 H.50** 1,0 H2O + HSO4 31.09 NaOH + H250. Na2SO4+H2O Part 1 Part 3 E s AH reaction Add the chemical reactions for parts 2 and 3. Do they give the same net chemical reaction as the reaction for part 1? Compare the average Act for part I with the sum of the results for parts 2 and 3 (pay attention to the number of significant figures). for part 1: kJ/mol Sum of the AH action for parts 2 and 3: kJ/mol Difference: _kJ/mol Calculate the percentage relative error for the difference between the two pathways difference percentage relative error = x 100% AH Are your results in agreement with Hess's law? Explain. 1. for parti (See Question 1 that follows for a discussion of the uncertainty in the measured values of the reaction enthalpies.) QUESTIONS 1. Measurement uncertainty. To get some idea of the uncertainty in your measured re- action enthalpies, repeat the calculation of the AH (in joules) for part 1(C). Trial 1, assuming that AT, is 0.4 C larger than the original one you measured. (We are assuming that the worst case uncertainty in ATwould be 0.4 C, because AT is the result of subtracting two temperature measurements, each having an uncertainty of 0.2 C when measured with a thermometer with 1 C intervals.) We are using only the AH term because it is the largest and most important term. The uncertainty in this term is essentially the same as the overall uncertainty in AH, calculated in part 1(g). Calculated values of AH. Goules): Original value calculated for ATC New value calculated for ATC +0.4 C reaction Now compare the new value to the old (first) value by calculating the relative percentage uncertainty in AH relative percentage uncertainty = AH (new) - AH (original) X 100% = AH (original) Typically, this relative percentage uncertainty is about 3%. Also keep in mind that our neglect of the heat losses to the calorimeter biases our results to values that are about 3% too low, 2. The enthalpy of dissociation of water. The H,SO, and NaOH reactants and the NaHSO, product in part 3 actually exist as dissociated ions, so the equation H,0* + HSO, +Na+ + OH-Na+ + HSO, +2 H,0 better represents the reaction (the total ionic equation). Write the equation that represents the net ionic equation by canceling out the spectator ions (those that appear on both sides of the total ionic equation). Net ionic equation: This net ionic equation represents the reaction of a strong acid and a strong base in water. Based on this result , and the measured a culo/mol from part 3, what is the approximate enthalpy change for the dissociation of water into H0 and OH-ions? (Hint: How is the net ionic reaction above related to the reaction below?) 2 H20 - H20(aq) + OH-(aq) Estimated . kJ/mol (Note: This is only an approximate estimate because HSO,-ion is nearly undissociated in 1 M H.SO, but is about 14% dissociated in 0.5 M NaHSO, because the enthalpy change for this partial dissociation is included in our measurements. Compare your estimate for the dissociation of water with the literature value of about +57 kJ/mol.) + reaction Enthalpy Changes in Chemical Reactions: Hess's Law DATA AND CALCULATIONS 1. The heat of solution and neutralization of 10 M H,SO, and 1.00 M NaOH M Trial 1 Trial 2 Data C 16.5 C 16.3 Initial temperature of 10 M H,SO, (5.0 mL in a graduated cylinder) 16.6 Initial temperature of calorimeter 1 16.2 (50.0 mL of 1.00 M NaOH + 45.0 mL water) Final temperature of the mixed solutions in calorimeter 1 (50 mL 1.00 M NaOH + 45.0 mL water + 5.0 mL 10 M H,SO.) 24.8 C 24.7 68 8.2 2722 J Calculations Trial 1 Trial 2 (a) Temperature change of NaOH solution in calorimeter 1 (AT-T- 18.6 .cv 8.40 (b) Temperature change of added H,So, solution (AT-T- "C Y.2 - (c) Use Equation (5) to calculate the heat gained by the NaOH solution. Assume that the product of density x heat capacity (1 763 (dp) is 4.10 JmL-KAHE- V.C. ATC J J (d) Use Equation (5) to calculate the heat gained by the H,SO solution. Assume that the product of density x heat capacity 167. 6 1681 (dC) is 4.10 3. ml. k. AH, - VdC, AT J (e) Calculate AH act by using the principle of conservation of energy expressed by Equation (6), rearranged to solve for the 1-1931.1 - 18901 enthalpy of reaction. AH Hati --AH-AH j J (6) Calculate the moles of added H,SO mol H.SO. - V. (L) X M (mol/L) a05 mol 0.05 mol (9) Calculate AH reaction per mole of reactants by dividing the results of part 10) by the number of moles of H,SO, calculated 39.62 37.9 in part 1(1). Express the final result in units of kJ/mol. Be sure to kJ/mol kJ/mol keep the proper sign of the calculated reaction enthalpy. Average -38.21 kJ/mol - ENTHALPY CHANGES IN CHEMICAL REACTIONS HESS'S LAW 2. The heat of solution of 10 MH,SO Data Trial 1 Trial 2 18.4 c 19.4 C C C Initial temperature of 10 M H,SO (10 mL in a graduated cylinder) Initial temperature of water in calorimeter 1 (90 mL delonized water) Final temperature of calorimeter 1 (90 mL water + added 10 mL H,SO) 16.3 16.7 23.9 C 23.2.c 69 Trial 2 Trial 1 c 6.5 " 7.6 " N 5.5. 4.8 " Calculations (a) Temperature change of water in calorimeter 1 (AT-T.-T.) (b) Temperature change of added H,SO, solution (AT. -T- (c) Use Equation (5) to calculate the heat gained by the NaOH solution. Use the same assumptions as in part 1 . = V dc. (d) Use Equation (5) to calculate the heat gained by the H,SO solution. AHA-V, dC, ATR 6.312 6.267 J 0.197 0.226 j - 6.538 -0.464 (e) Calculate AH Heraction by using the principle of conservation of energy expressed by Equation (6), rearranged to solve for the enthalpy of reaction Action = -A Hc- AHA J 6.1 0.1 mol mol (f) Calculate the moles of added H, SO, mol H,SO. - V (L) X M (mol/L) -5.38 -4.64 (g) Calculate AH.nection per mole of reactants by dividing the results of part 2(e) by the number of moles of H.SO, calculated in part 2(1). Express the final result in units of kJ/mol. Be sure to keep the proper sign of the calculated reaction enthalpy. kJ/mol kJ/mol kJ/mol -OGOSOL Average 3. The heat of neutralization of 1.00 MH,SO and 1.00 M NaOH Trial 1 Trial 2 Data 116.6 16.a C C C C 16.3 Initial temperature of NaOH solution in calorimeter 1 (50 mL of 1.00 M NaOH) Initial temperature of H,SO, solution in calorimeter 2 (50 mL of 1.00 M H,SO) Final temperature of mixture in calorimeter 1 (50 mL 1.00 M NaOH + 50 mL 1.00 MH,SO.) 16.2 23.4 23.8 C C Trial 1 Trial 2 C Calculations (a) Temperature change of NaOH solution in calorimeter 1 (AT= = Tina - Tinitia) (b) Temperature change of added H,SO, solution (ATA - Tina - Toni (c) Use Equation (5) to calculate the heat gained by the NaOH solution. Use the same assumptions as in part 2. AHC = V dC, ATC 0.8 7.2 C 7.5 C 0.2829 10 2788 J J (d) Calculate the heat gained by the H,SO, solution AHR = VDC ATR 10 295 0.3075 J -0.31365 (e) Calculate AH reaction by using the principle of conservation of energy expressed by Equation (5), rearranged to solve for the -0.3083 enthalpy of reaction. AH reaction --A Hc- AHA J J (1) Calculate the moles of added H,SO, mol H,SO, EVA(L) X M (mol/L) 0.1 mol 0.1 mol 0.003083 6.00 31365 (g) Calculate AH reaction per mole of reactants by dividing the results of part 3(e) by the number of moles of H, 50, calculated in part 3(1). Express the final result in units of kJ/mol. Be sure to keep the proper sign of the calculated reaction enthalpy. kJ/mol kJ/mol Average 0.00310075 kJ/mol 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


