Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Not sure how to do this spectrophotometric table 2.4. please show all steps for the stalk solution and solution A. thanks 1) Calculations for the
Not sure how to do this spectrophotometric table 2.4. please show all steps for the stalk solution and solution A. thanks 
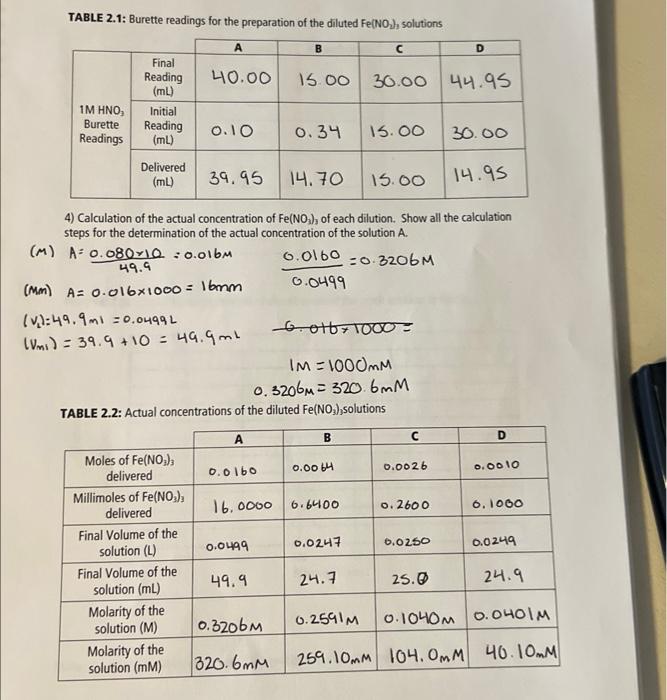
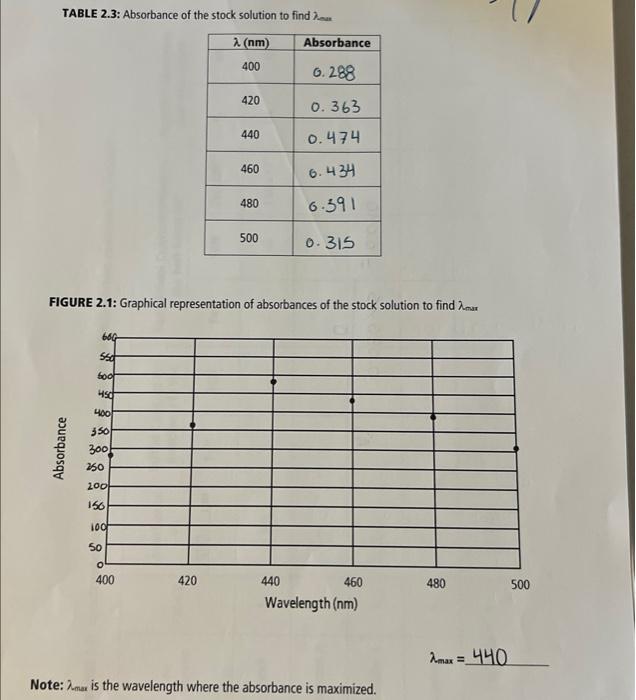
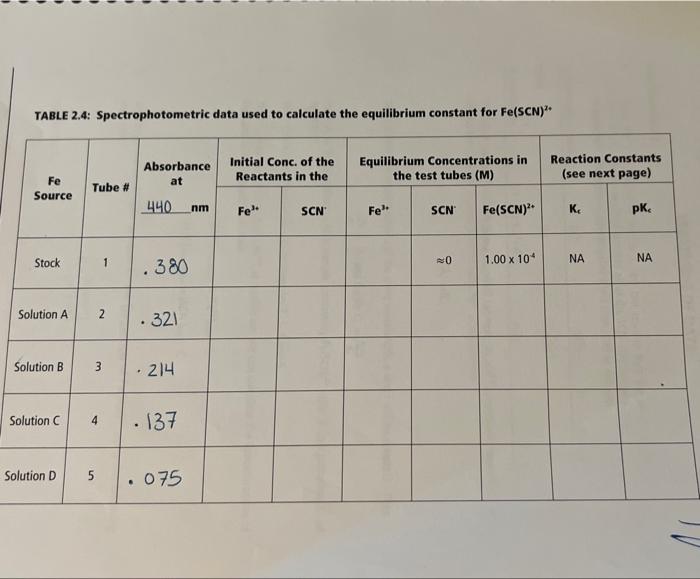
1) Calculations for the Fe(NO3)3 solution 0.200M50mL0.0100403.8g1090O=4.038g 0.05kx0.200m=0.0100m Calculated mass of Fe(NO3)9H2O:4.038g Actual mass of Fe(NO3) ): 9H2O:4.049g Calculations for the actual concentration. c=503.8g/mol4.049g=0.0100Ac=50L4.049g=0.080g12 A : Actual concentration of Fe(NO3)3:0.080g/L TABLE 2.1: Burette readings for the preparation of the diluted Fe(NO 2)3, solutions 4) Calculation of the actual concentration of Fe(NO3)3 of each dilution. Show all the calculation steps for the determination of the actual concentration of the solution A. (M)A=49.90.08010=0.016M(Mm)A=0.0161000=16mm0.04990.0160=0.3206M=0.01(vL)=49.9ml=0.0499L(Vm1)=39.9+10=49.9ml6.016+1000=IM=1000OmM0.3206M=320.6mm TABLE 2.2: Actual concentrations of the diluted Fe(NO3),solutions TABLE 2.3: Absorbance of the stock solution to find an FIGURE 2.1: Graphical representation of absorbances of the stock solution to find max max=44 Note: mar is the wavelength where the absorbance is maximized. TABLE 2.4: Spectrophotometric data used to calculate the equilibrium constant for Fe(SCN) 2 1) Calculations for the Fe(NO3)3 solution 0.200M50mL0.0100403.8g1090O=4.038g 0.05kx0.200m=0.0100m Calculated mass of Fe(NO3)9H2O:4.038g Actual mass of Fe(NO3) ): 9H2O:4.049g Calculations for the actual concentration. c=503.8g/mol4.049g=0.0100Ac=50L4.049g=0.080g12 A : Actual concentration of Fe(NO3)3:0.080g/L TABLE 2.1: Burette readings for the preparation of the diluted Fe(NO 2)3, solutions 4) Calculation of the actual concentration of Fe(NO3)3 of each dilution. Show all the calculation steps for the determination of the actual concentration of the solution A. (M)A=49.90.08010=0.016M(Mm)A=0.0161000=16mm0.04990.0160=0.3206M=0.01(vL)=49.9ml=0.0499L(Vm1)=39.9+10=49.9ml6.016+1000=IM=1000OmM0.3206M=320.6mm TABLE 2.2: Actual concentrations of the diluted Fe(NO3),solutions TABLE 2.3: Absorbance of the stock solution to find an FIGURE 2.1: Graphical representation of absorbances of the stock solution to find max max=44 Note: mar is the wavelength where the absorbance is maximized. TABLE 2.4: Spectrophotometric data used to calculate the equilibrium constant for Fe(SCN) 2 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


