Answered step by step
Verified Expert Solution
Question
1 Approved Answer
not sure how to do this What mass of NaHCO3(s) must have been present at the beginning of the reaction? Hide Hint Hint: Your goal
not sure how to do this 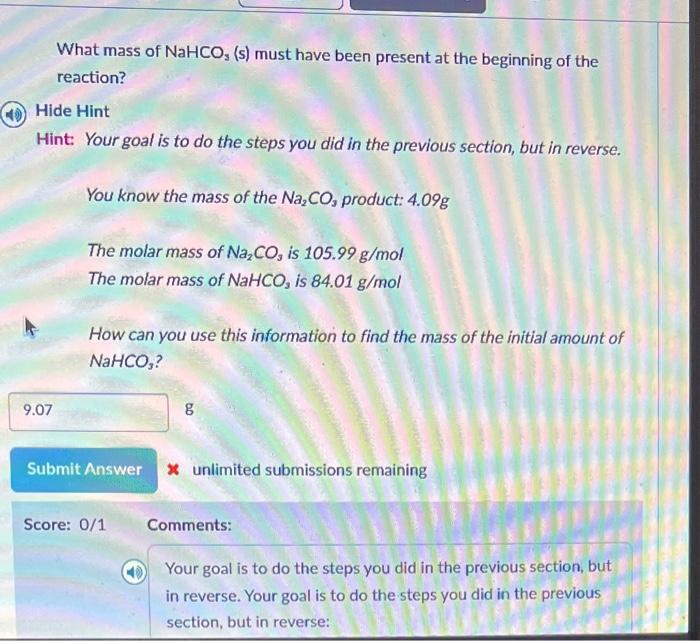
What mass of NaHCO3(s) must have been present at the beginning of the reaction? Hide Hint Hint: Your goal is to do the steps you did in the previous section, but in reverse. You know the mass of the Na2CO3 product: 4.09g The molar mass of Na2CO3 is 105.99g/mol The molar mass of NaHCO3 is 84.01g/mol How can you use this information to find the mass of the initial amount of NaHCOs ? g x unlimited submissions remaining Score: 0/1 Comments: Your goal is to do the steps you did in the previous section, but in reverse. Your goal is to do the steps you did in the previous section, but in reverse 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


