Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Note: 75% azo dye was extracted after two times extractions with 50ml NaCl. it is much appreciated if all questions could be answered, thank you
Note: 75% azo dye was extracted after two times extractions with 50ml NaCl. it is much appreciated if all questions could be answered, thank you
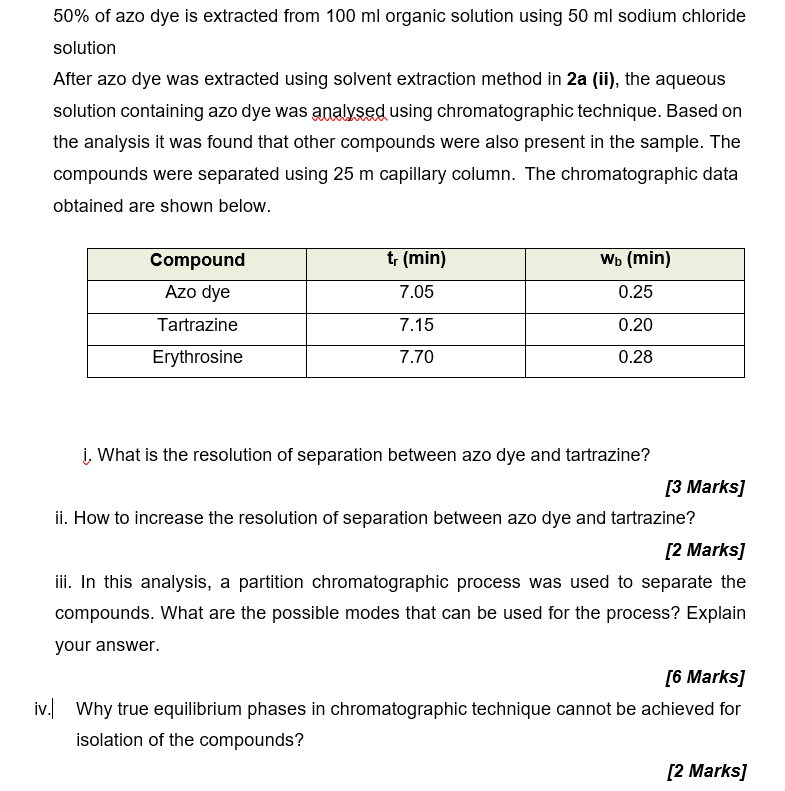
50% of azo dye is extracted from 100 ml organic solution using 50 ml sodium chloride solution After azo dye was extracted using solvent extraction method in 2a (ii), the aqueous solution containing azo dye was analysed using chromatographic technique. Based on the analysis it was found that other compounds were also present in the sample. The compounds were separated using 25 m capillary column. The chromatographic data obtained are shown below. ty (min) WD (min) Compound Azo dye Tartrazine 7.05 0.25 7.15 0.20 Erythrosine 7.70 0.28 , What is the resolution of separation between azo dye and tartrazine? [3 Marks] ii. How to increase the resolution of separation between azo dye and tartrazine? [2 Marks] iii. In this analysis, a partition chromatographic process was used to separate the compounds. What are the possible modes that can be used for the process? Explain your answer. [6 Marks] iv. Why true equilibrium phases in chromatographic technique cannot be achieved for isolation of the compounds? [2 Marks]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


