Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Note: for this homework, all thermodynamic properties must be retrieved using the tables in your textbook. Please include the table number you used. Problem
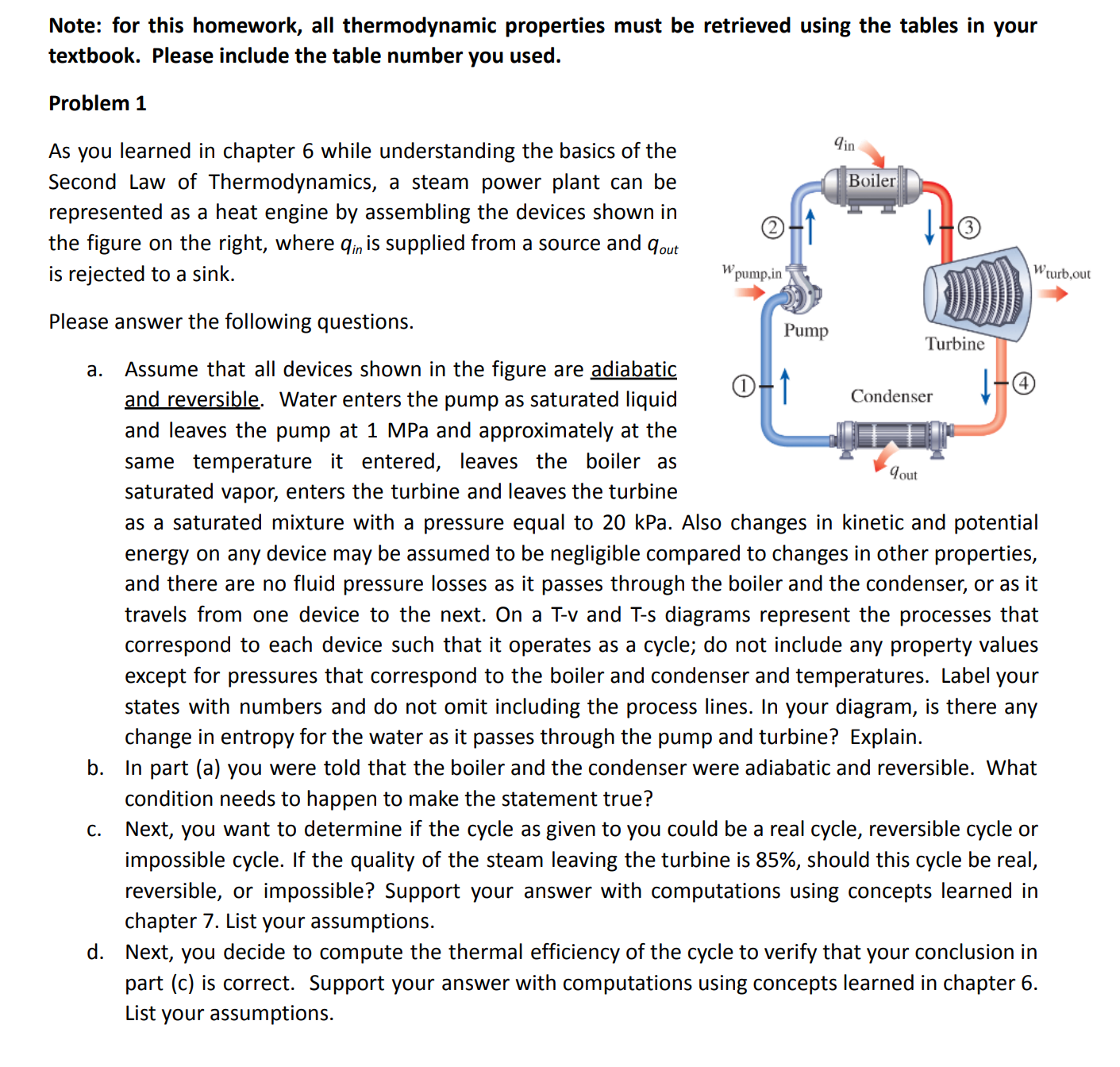
Note: for this homework, all thermodynamic properties must be retrieved using the tables in your textbook. Please include the table number you used. Problem 1 As you learned in chapter 6 while understanding the basics of the Second Law of Thermodynamics, a steam power plant can be represented as a heat engine by assembling the devices shown in the figure on the right, where qin is supplied from a source and Yout is rejected to a sink. Please answer the following questions. W pump,in Pump 0-1 Jin Boiler Turbine 1-4 Condenser Yout Wturb,out a. Assume that all devices shown in the figure are adiabatic and reversible. Water enters the pump as saturated liquid and leaves the pump at 1 MPa and approximately at the same temperature it entered, leaves the boiler as saturated vapor, enters the turbine and leaves the turbine as a saturated mixture with a pressure equal to 20 kPa. Also changes in kinetic and potential energy on any device may be assumed to be negligible compared to changes in other properties, and there are no fluid pressure losses as it passes through the boiler and the condenser, or as it travels from one device to the next. On a T-v and T-s diagrams represent the processes that correspond to each device such that it operates as a cycle; do not include any property values except for pressures that correspond to the boiler and condenser and temperatures. Label your states with numbers and do not omit including the process lines. In your diagram, is there any change in entropy for the water as it passes through the pump and turbine? Explain. b. In part (a) you were told that the boiler and the condenser were adiabatic and reversible. What condition needs to happen to make the statement true? c. Next, you want to determine if the cycle as given to you could be a real cycle, reversible cycle or impossible cycle. If the quality of the steam leaving the turbine is 85%, should this cycle be real, reversible, or impossible? Support your answer with computations using concepts learned in chapter 7. List your assumptions. d. Next, you decide to compute the thermal efficiency of the cycle to verify that your conclusion in part (c) is correct. Support your answer with computations using concepts learned in chapter 6. List your assumptions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


