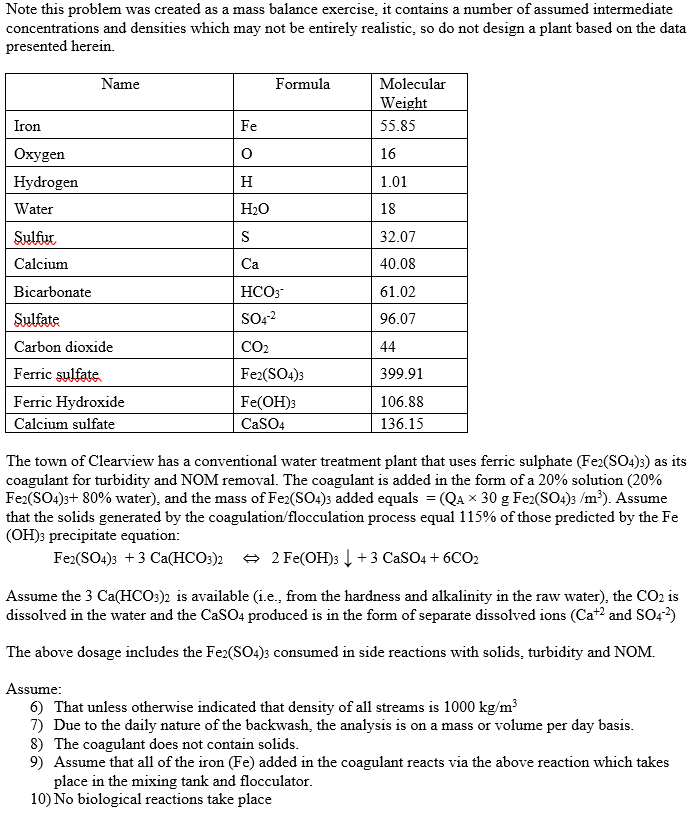

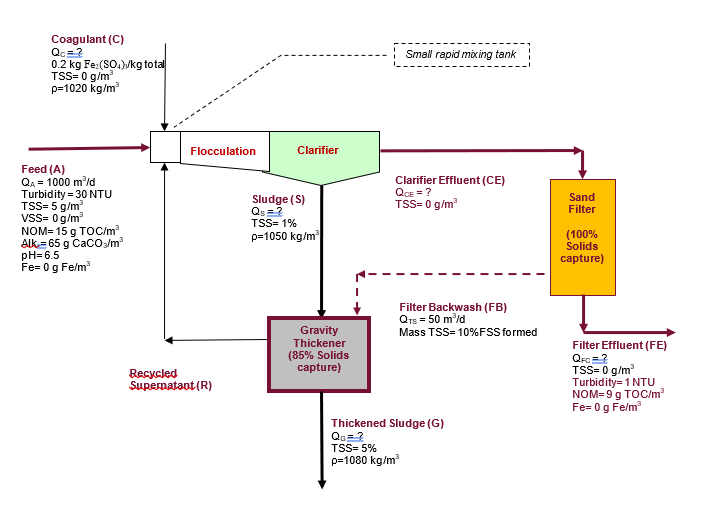
Note this problem was created as a mass balance exercise, it contains a number of assumed intermediate concentrations and densities which may not be entirely realistic, so do not design a plant based on the data presented herein. Name Formula Molecular Weight 55.85 Iron Fe o 16 Oxygen Hydrogen Water H 1.01 H2O 18 S 32.07 Ca 40.08 61.02 HCO3- SO42 96.07 Sulfur Calcium Bicarbonate Sulfate Carbon dioxide Ferric sulfate Ferric Hydroxide Calcium sulfate 44 399.91 CO2 Fe2(SO4)3 Fe(OH)3 CaSO4 106.88 136.15 The town of Clearview has a conventional water treatment plant that uses ferric sulphate (Fe2(SO4)3) as its coagulant for turbidity and NOM removal. The coagulant is added in the form of a 20% solution (20% Fe2(SO4)3+ 80% water), and the mass of Fe2(SO4)3 added equals = (QA * 30 g Fe2(SO4)3 /m?). Assume that the solids generated by the coagulation/flocculation process equal 115% of those predicted by the Fe (OH)3 precipitate equation: Fe2(SO4)3 + 3 Ca(HCO3)2 2 Fe(OH)3 | +3 CaSO4 +6CO2 Assume the 3 Ca(HCO3)2 is available (i.e., from the hardness and alkalinity in the raw water), the CO2 is dissolved in the water and the CaSO4 produced is in the form of separate dissolved ions (Ca+2 and S042) The above dosage includes the Fe2(SO4)3 consumed in side reactions with solids, turbidity and NOM. Assume: 6) That unless otherwise indicated that density of all streams is 1000 kg/m3 7) Due to the daily nature of the backwash, the analysis is on a mass or volume per day basis. 8) The coagulant does not contain solids. 9) Assume that all of the iron (Fe) added in the coagulant reacts via the above reaction which takes place in the mixing tank and flocculator. 10) No biological reactions take place 11) Some of the Fe(OH)3 particles formed are too small to be considered FSS when they reach the clarifier, however they manage to become large enough in the downstream sand filter. Assume that the effluent of the clarifier has no TSS, 10% of the suspended solids formed are actually formed and temporarily trapped in the sand filter. As the sand filter captures 100% of the suspended solids upon the daily backwashing all these solids (10% of the FSS formed) also return the gravity thickener. Therefore, 90% of the FSS is formed in the flocculation tank and 10% is formed in the sand filter. 12) The clarifier is a solids separations process, it does not separate dissolved species. 13) The sand filter is primarily a solids separations process but 10% of the FSS is formed there. Dissolved species are not separated by the filter. 14) The gravity thickener is strictly a solids separation process, not a reactor. 15) The sludges from the primary clarifier and filter backwash are concentrated in a gravity thickener which has a solids capture of 85%. NOTE: 1) The solids capture is primarily used for sludge treatment processes, like the gravity thickener in the problem. The solids capture is the percent of the solids (TSS) in the influents(s) to the process that end up in the solids rich exit stream. In this particular problem you have two inlet streams (S and FB) and the solids rich stream is G. Solids capture for the gravity thickener = = 100 * (Qg*99*XGTSS)/ [(Qs*es*X$TSS)+(Mass of TSS in FB)] 2) This problem also uses the solids capture for the sand filter Determine: a) Volumetric flowrate of the coagulant solution (Qc) in m/d b) Volume of flowrate of thickened sludge (G) in m/d. (If you are unable to solve part a) assume Qc = 1 m/d). c) The volumetric flowrate of sludge (S) in m3/d. (If you are unable to solve part b) assume Qg = 0.5 m/d). d) The TSS concentration of the recycle stream (R) in m/d. (If you are unable to solve part c) assume Qs =1 m/d). = Small rapid mixing tank Coagulant (C) Qc = ? 0.2 kg Fe (SOx)/kg total TSS=0 g/m p=1020 kg/m2 Flocculation Clarifier = Clarifier Effluent (CE) QCE = ? TSS=0 g/m Sand Filter Feed (A) QA = 1000 m/d Turbidity = 30 NTU TSS=5 g/m VSS=0 g/m NOM=15g TOC/m Alk=65 g CaCO3/m pH=6.5 Fe=0g Fe/m Sludge(s) Qs = ? TSS= 1% p=1050 kg/m (100% Solids capture) Filter Backwash (FB) Qts = 50 mld Mass TSS=10%FSS formed Gravity Thickener (85% Solids capture) Recycled Supernatant (R) Filter Effluent (FE) QFc = ? TSS=0 g/m Turbidity= 1 NTU NOM=9g TOC/m Fe= 0 g Fe/m Thickened Sludge (G) Q. = ? TSS= 5% p=1080 kg/m









