Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13. The nuclide Th234 (UX) is normally present in uranium compounds in secular equilibrium with its parent U238, which has a half-life of 4.50
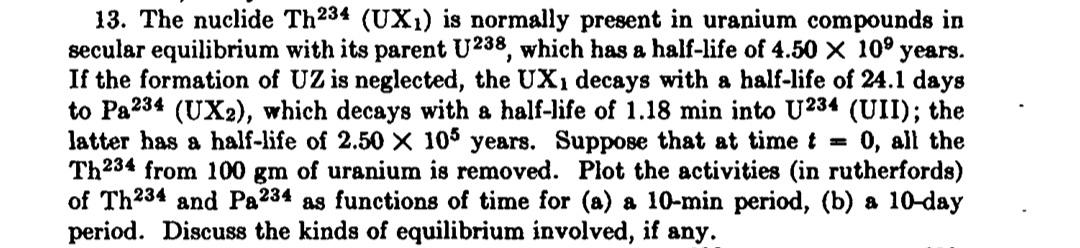
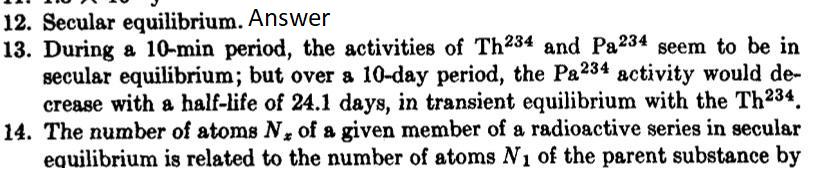
13. The nuclide Th234 (UX) is normally present in uranium compounds in secular equilibrium with its parent U238, which has a half-life of 4.50 10 years. If the formation of UZ is neglected, the UX1 decays with a half-life of 24.1 days to Pa234 (UX2), which decays with a half-life of 1.18 min into U234 (UII); the latter has a half-life of 2.50 X 105 years. Suppose that at time t = 0, all the Th234 from 100 gm of uranium is removed. Plot the activities (in rutherfords) of Th234 and Pa234 as functions of time for (a) a 10-min period, (b) a 10-day period. Discuss the kinds of equilibrium involved, if any. 12. Secular equilibrium. Answer 13. During a 10-min period, the activities of Th234 and Pa234 seem to be in secular equilibrium; but over a 10-day period, the Pa234 activity would de- crease with a half-life of 24.1 days, in transient equilibrium with the Th234. 14. The number of atoms N, of a given member of a radioactive series in secular equilibrium is related to the number of atoms N of the parent substance by
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Solution present in uranium with its 450 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


