Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) Draw an electron-pushing mechanism for the formation of the propionylium cation resulting from the reaction of propionyl chloride and FeCl3. Show all lone
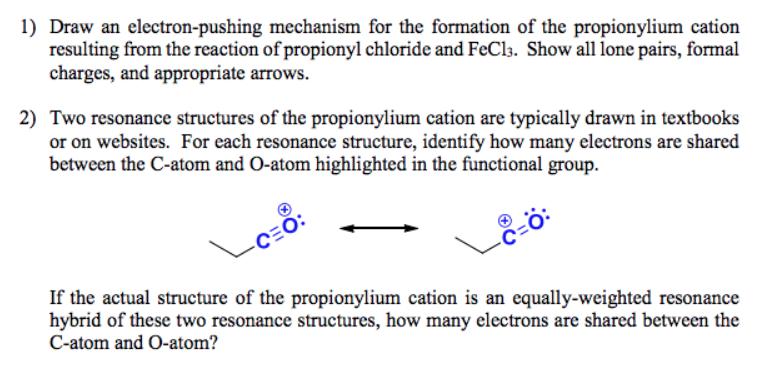
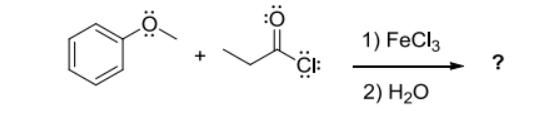
1) Draw an electron-pushing mechanism for the formation of the propionylium cation resulting from the reaction of propionyl chloride and FeCl3. Show all lone pairs, formal charges, and appropriate arrows. 2) Two resonance structures of the propionylium cation are typically drawn in textbooks or on websites. For each resonance structure, identify how many electrons are shared between the C-atom and O-atom highlighted in the functional group. CEC If the actual structure of the propionylium cation is an equally-weighted resonance hybrid of these two resonance structures, how many electrons are shared between the C-atom and O-atom? CE 1) FeCl3 2) HO ?
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER CI FeCl3 6 e...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


