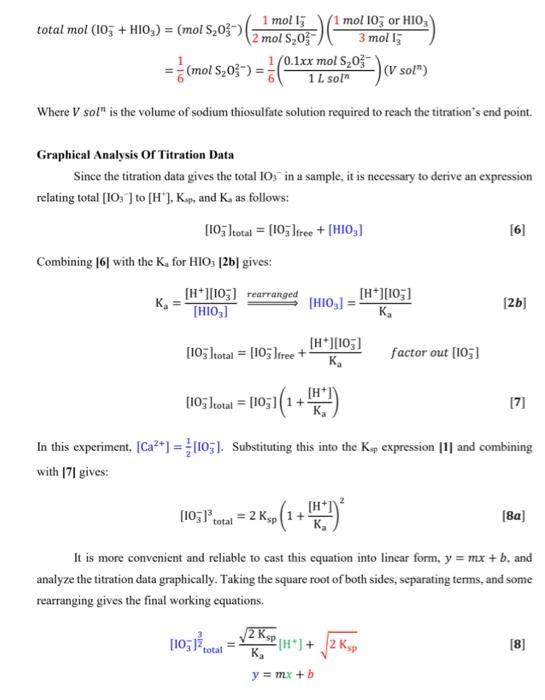
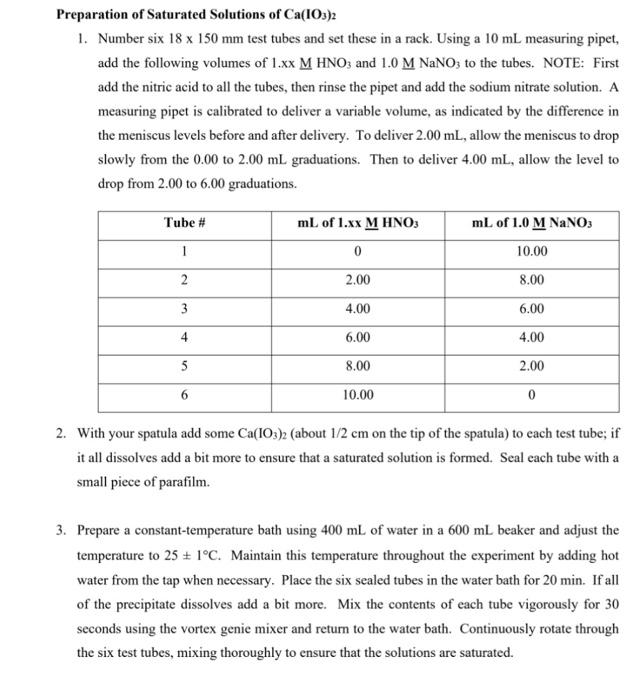
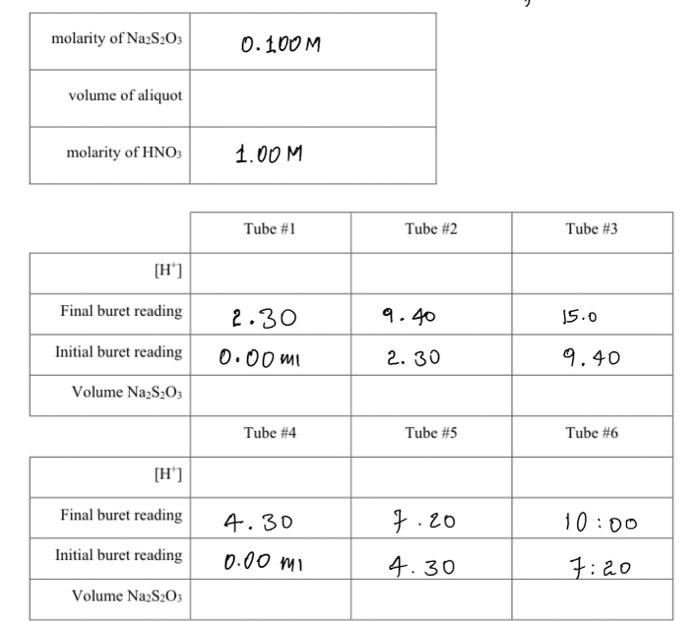
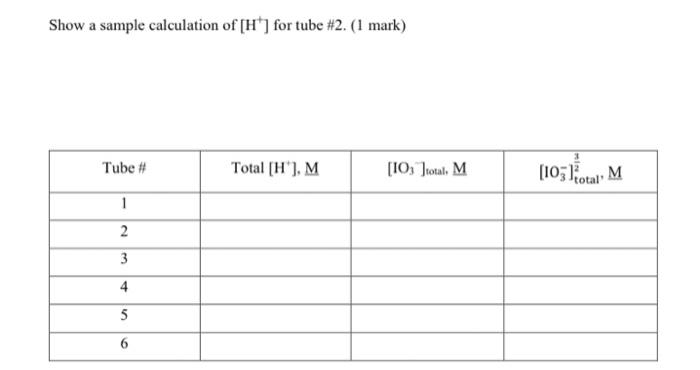
OBJECTIVES: In this experiment you will investigate the solubility of calcium iodate in solutions of varying amounts of added nitric acid (i.e. varying [H3O+]) and determine the solubility product constant Ksp of calcium iodate, and the acid dissociation constant Ka of iodic acid, HIO3. This experiment also illustrates graphical analysis of data. In this experiment, you will get hands-on experience determining Ksp (Unit 2 ) as well as applying your knowledge of Ka from Unit 1 (and unit 10 from CHEM 211/203). In addition, this is a real-life application of simultaneous equilibria discussed throughout the last units of CHEM 211/203 and the first few units of CHEM 212/205. THEORY: A sparingly soluble salt is in equilibrium with its ions in a saturated solution of the salt. In general, for a salt MnXm the solubility equilibrium may be expressed as: MnXm(s)KspnMm+(aq)+mXn(aq) and the equilibrium constant for this reaction is called the solubility product constant, Ksp ? Ksp=[Mm+]n[Xn]m In the case of calcium iodate, the solubility equilibrium and the Ksp expression is: Ca(IO3)2(s)Ca2+(aq)+2IO3(aq)Ksp=[Ca2+][IO3]2 Since iodate ion is the conjugate base of iodic acid (HIO3) which is a weak acid, the solubility of calcium iodate is greatly enhanced in acid solution. At higher [H+]more of the IO3is protonated and thus the solubility equilibrium of calcium iodate (equation [1]) is shifted to the right. Combining the two interactive equilibria ( Ksp of calcium iodate and Ka of periodic acid) involved we have reaction [2] below: 1 Ca(IO3)2(s)Ca2+(aq)+2103(aq)K5pp[2a]2{H+(aq)+IO3(aq)HIO3(aq)}(Ka)21Ca(IO3)2(s)+2H+(aq)Ca2+(aq)+2HIO3(aq)Koverall=(Ka)2Ksp EXPERIMENTAL METHOD: You will prepare a series of saturated solutions of Ca(IO3)2, each at a different concentration of added HNO3, by adding excess solid Ca(IO3)2 to solutions of HNO3 and NaNO3 and then shaking the mixture to establish the two interactive equilibria summarized by Equation (2). The NaNO3, which does not enter into the reaction, ensures a constant total ion concentration since equilibrium constants in general depend somewhat on solution ionic strength. You will remove the saturated supernatant liquid from the excess solid Ca(IO5)2 in each test tube and titrate a constant aliquot of each to determine the total IO3concentration. Note that since iodic acid HIO3 is a weak acid, iodate is present partly as free IO3but mostly locked up in the form of the conjugate acid HIO3 in the acidified solutions, i.e. [IO3]total=[IO3]free+[IO3]asHo The total iodate concentration in each solution will be determined by adding HCl and an excess of KI to an aliquot of the sample. In the ensuing redox reaction, all iodate (IO3+HIO3) is quantitatively reduced by iodide to form I2, which in the presence of excess Iforms I3ion. Triiodide ion causes the solution to be reddish-brown color at this stage. IO3(aq)+6H+(aq)+8I(aq)3I3(aq)+3H2O(l) The released iodine as I3is then titrated with a standard solution of sodium thiosulfate using starch as indicator. I3(aq)+2S2O32(aq)3I(aq)+S4O62(aq) The endpoint for this titration is the disappearance of the intense blue color of the starchiodine complex. At the stoichiometric equivalence point of the titration, =61(molS2O32)=61(1Lsoln0.1xxmolS2O32)(Vsoln) Where V sol ln is the volume of sodium thiosulfate solution required to reach the titration's end point. Graphical Analysis Of Titration Data Since the titration data gives the total IO3in a sample, it is necessary to derive an expression relating total [IO3]to [H+],Ksp, and K as follows: [IO3]total=[IO3]free+[HIO3] Combining [6] with the Ka for HIO3[2b] gives: Ka=[HIO3][H+][IO3]=rearranged[HIO3]=Ka[H+][IO3][IO3]total=[IO3]free+Ka[H+][103]factorout[IO3][103]total=[103](1+Ka[H+]) In this experiment, [Ca2+]=21[IO3]. Substituting this into the Ks expression [1] and combining with [7] gives: [103]total3=2Ksp(1+Ka[H+])2 It is more convenient and reliable to cast this equation into linear form, y=mx+b, and analyze the titration data graphically. Taking the square root of both sides, separating terms, and some rearranging gives the final working equations. [103]23total=Ka2Ksp[H+]+2Kspy=mx+b Therefore, a graph of [IO3]23 vs. [H+]should yield a straight line with slope =m=Ka2Ksp and y intercept =b=2Ksp. From the measured slope and y-intercept, it follows that: b=2KspKsp=2b2m=Ka2Ksp=KabKa=mb Figure 1: Solubility of Ca(IO3)2 under Varying Acid Conditions This technique of transforming a seemingly complicated equation into a linear relationship is commonly used in chemistry to obtain the constant parameters, in this case Ksp of Ca(IO3)2 and Ka HIO3. REAGENTS: Ca(IO3)2(aq) (must be freshly prepared) 1.xx MHNO3 (standardized) 0.1xMNa2S2O3 (standardized) KI(s), starch solution, 1.0MNaNO3,1MHCl Preparation of Saturated Solutions of Ca(IO3)2 1. Number six 18150mm test tubes and set these in a rack. Using a 10mL measuring pipet, add the following volumes of 1.xxMHNO3 and 1.0MNaNO3 to the tubes. NOTE: First add the nitric acid to all the tubes, then rinse the pipet and add the sodium nitrate solution. A measuring pipet is calibrated to deliver a variable volume, as indicated by the difference in the meniscus levels before and after delivery. To deliver 2.00mL, allow the meniscus to drop slowly from the 0.00 to 2.00mL graduations. Then to deliver 4.00mL, allow the level to drop from 2.00 to 6.00 graduations. it all dissolves add a bit more to ensure that a saturated solution is formed. Seal each tube with a small piece of parafilm. 3. Prepare a constant-temperature bath using 400mL of water in a 600mL beaker and adjust the temperature to 251C. Maintain this temperature throughout the experiment by adding hot water from the tap when necessary. Place the six sealed tubes in the water bath for 20min. If all of the precipitate dissolves add a bit more. Mix the contents of each tube vigorously for 30 seconds using the vortex genie mixer and return to the water bath. Continuously rotate through the six test tubes, mixing thoroughly to ensure that the solutions are saturated. \begin{tabular}{|r|l|} \hline molarity of Na2S2O3 & 0.100M \\ \hline volume of aliquot & \\ \hline molarity of HNO3 & 1.00M \\ \hline \end{tabular} \begin{tabular}{|r|c|c|c|} \cline { 2 - 5 } \multicolumn{1}{c|}{} & \multicolumn{1}{c|}{ Tube \#1 } & Tube #2 & Tube \#3 \\ \hline[H+] & & & \\ \hline Final buret reading & 2.30 & 9.40 & 15.0 \\ \hline Initial buret reading & 0.00ml & 2.30 & 9.40 \\ \hline Volume Na2S2O3 & & & \\ \hline & Tube #4 & Tube #5 & Tube \#6 \\ \hline Final buret reading & 4.30 & 7.20 & 10:00 \\ \hline Initial buret reading & 0.00m1 & 4.30 & 7.20 \\ \hline Volume Na2S2O3 & & & \\ \hline \end{tabular} 1. From your titration data, calculate the total concentration of iodate in each saturated solution. See advance study question \#2. Show calculations for tube \#1 and tabulate the results for the six samples below. Also calculate [IO3]23 for each sample. NOTE: Use xy key on your calculator. Show a sample calculation of [H+]for tube \#2. ( 1 mark) 2. Plot a graph of [IO3]total2vv. [H+]and draw the best-fit straight line through the points. Refer to I\&A "Graphing Requirements". Attach the labeled graph to your report. (3 marks) 3. Determine the slope and y-intercept when [H]=0. Show calculations on your graph. ( 2 marks) 4. Calculate the Ksp of Ca(IO3)2 and the K2 of HIO3. Refer to equations [8] through [10]. Show calculations below. ( 2 marks) 5. Calculate the % error in your values of Ksp and Ka compared to the following values obtained by previous lab sections: Ksp=3.3105;Ka=0.48 at 25C (1 mark) 6. Explain how the following determinate errors would affect your calculated value for Ksp. a) The calcium iodate solutions were not completely saturated. ( 1 mark) b) Some solid Ca(IO3)2 was present in the aliquot of filtrate used for analysis. (1 mark) 7. What appears to be the largest source of determinate error in your experimental determination of the Ksp ? Explain. (1 mark) OBJECTIVES: In this experiment you will investigate the solubility of calcium iodate in solutions of varying amounts of added nitric acid (i.e. varying [H3O+]) and determine the solubility product constant Ksp of calcium iodate, and the acid dissociation constant Ka of iodic acid, HIO3. This experiment also illustrates graphical analysis of data. In this experiment, you will get hands-on experience determining Ksp (Unit 2 ) as well as applying your knowledge of Ka from Unit 1 (and unit 10 from CHEM 211/203). In addition, this is a real-life application of simultaneous equilibria discussed throughout the last units of CHEM 211/203 and the first few units of CHEM 212/205. THEORY: A sparingly soluble salt is in equilibrium with its ions in a saturated solution of the salt. In general, for a salt MnXm the solubility equilibrium may be expressed as: MnXm(s)KspnMm+(aq)+mXn(aq) and the equilibrium constant for this reaction is called the solubility product constant, Ksp ? Ksp=[Mm+]n[Xn]m In the case of calcium iodate, the solubility equilibrium and the Ksp expression is: Ca(IO3)2(s)Ca2+(aq)+2IO3(aq)Ksp=[Ca2+][IO3]2 Since iodate ion is the conjugate base of iodic acid (HIO3) which is a weak acid, the solubility of calcium iodate is greatly enhanced in acid solution. At higher [H+]more of the IO3is protonated and thus the solubility equilibrium of calcium iodate (equation [1]) is shifted to the right. Combining the two interactive equilibria ( Ksp of calcium iodate and Ka of periodic acid) involved we have reaction [2] below: 1 Ca(IO3)2(s)Ca2+(aq)+2103(aq)K5pp[2a]2{H+(aq)+IO3(aq)HIO3(aq)}(Ka)21Ca(IO3)2(s)+2H+(aq)Ca2+(aq)+2HIO3(aq)Koverall=(Ka)2Ksp EXPERIMENTAL METHOD: You will prepare a series of saturated solutions of Ca(IO3)2, each at a different concentration of added HNO3, by adding excess solid Ca(IO3)2 to solutions of HNO3 and NaNO3 and then shaking the mixture to establish the two interactive equilibria summarized by Equation (2). The NaNO3, which does not enter into the reaction, ensures a constant total ion concentration since equilibrium constants in general depend somewhat on solution ionic strength. You will remove the saturated supernatant liquid from the excess solid Ca(IO5)2 in each test tube and titrate a constant aliquot of each to determine the total IO3concentration. Note that since iodic acid HIO3 is a weak acid, iodate is present partly as free IO3but mostly locked up in the form of the conjugate acid HIO3 in the acidified solutions, i.e. [IO3]total=[IO3]free+[IO3]asHo The total iodate concentration in each solution will be determined by adding HCl and an excess of KI to an aliquot of the sample. In the ensuing redox reaction, all iodate (IO3+HIO3) is quantitatively reduced by iodide to form I2, which in the presence of excess Iforms I3ion. Triiodide ion causes the solution to be reddish-brown color at this stage. IO3(aq)+6H+(aq)+8I(aq)3I3(aq)+3H2O(l) The released iodine as I3is then titrated with a standard solution of sodium thiosulfate using starch as indicator. I3(aq)+2S2O32(aq)3I(aq)+S4O62(aq) The endpoint for this titration is the disappearance of the intense blue color of the starchiodine complex. At the stoichiometric equivalence point of the titration, =61(molS2O32)=61(1Lsoln0.1xxmolS2O32)(Vsoln) Where V sol ln is the volume of sodium thiosulfate solution required to reach the titration's end point. Graphical Analysis Of Titration Data Since the titration data gives the total IO3in a sample, it is necessary to derive an expression relating total [IO3]to [H+],Ksp, and K as follows: [IO3]total=[IO3]free+[HIO3] Combining [6] with the Ka for HIO3[2b] gives: Ka=[HIO3][H+][IO3]=rearranged[HIO3]=Ka[H+][IO3][IO3]total=[IO3]free+Ka[H+][103]factorout[IO3][103]total=[103](1+Ka[H+]) In this experiment, [Ca2+]=21[IO3]. Substituting this into the Ks expression [1] and combining with [7] gives: [103]total3=2Ksp(1+Ka[H+])2 It is more convenient and reliable to cast this equation into linear form, y=mx+b, and analyze the titration data graphically. Taking the square root of both sides, separating terms, and some rearranging gives the final working equations. [103]23total=Ka2Ksp[H+]+2Kspy=mx+b Therefore, a graph of [IO3]23 vs. [H+]should yield a straight line with slope =m=Ka2Ksp and y intercept =b=2Ksp. From the measured slope and y-intercept, it follows that: b=2KspKsp=2b2m=Ka2Ksp=KabKa=mb Figure 1: Solubility of Ca(IO3)2 under Varying Acid Conditions This technique of transforming a seemingly complicated equation into a linear relationship is commonly used in chemistry to obtain the constant parameters, in this case Ksp of Ca(IO3)2 and Ka HIO3. REAGENTS: Ca(IO3)2(aq) (must be freshly prepared) 1.xx MHNO3 (standardized) 0.1xMNa2S2O3 (standardized) KI(s), starch solution, 1.0MNaNO3,1MHCl Preparation of Saturated Solutions of Ca(IO3)2 1. Number six 18150mm test tubes and set these in a rack. Using a 10mL measuring pipet, add the following volumes of 1.xxMHNO3 and 1.0MNaNO3 to the tubes. NOTE: First add the nitric acid to all the tubes, then rinse the pipet and add the sodium nitrate solution. A measuring pipet is calibrated to deliver a variable volume, as indicated by the difference in the meniscus levels before and after delivery. To deliver 2.00mL, allow the meniscus to drop slowly from the 0.00 to 2.00mL graduations. Then to deliver 4.00mL, allow the level to drop from 2.00 to 6.00 graduations. it all dissolves add a bit more to ensure that a saturated solution is formed. Seal each tube with a small piece of parafilm. 3. Prepare a constant-temperature bath using 400mL of water in a 600mL beaker and adjust the temperature to 251C. Maintain this temperature throughout the experiment by adding hot water from the tap when necessary. Place the six sealed tubes in the water bath for 20min. If all of the precipitate dissolves add a bit more. Mix the contents of each tube vigorously for 30 seconds using the vortex genie mixer and return to the water bath. Continuously rotate through the six test tubes, mixing thoroughly to ensure that the solutions are saturated. \begin{tabular}{|r|l|} \hline molarity of Na2S2O3 & 0.100M \\ \hline volume of aliquot & \\ \hline molarity of HNO3 & 1.00M \\ \hline \end{tabular} \begin{tabular}{|r|c|c|c|} \cline { 2 - 5 } \multicolumn{1}{c|}{} & \multicolumn{1}{c|}{ Tube \#1 } & Tube #2 & Tube \#3 \\ \hline[H+] & & & \\ \hline Final buret reading & 2.30 & 9.40 & 15.0 \\ \hline Initial buret reading & 0.00ml & 2.30 & 9.40 \\ \hline Volume Na2S2O3 & & & \\ \hline & Tube #4 & Tube #5 & Tube \#6 \\ \hline Final buret reading & 4.30 & 7.20 & 10:00 \\ \hline Initial buret reading & 0.00m1 & 4.30 & 7.20 \\ \hline Volume Na2S2O3 & & & \\ \hline \end{tabular} 1. From your titration data, calculate the total concentration of iodate in each saturated solution. See advance study question \#2. Show calculations for tube \#1 and tabulate the results for the six samples below. Also calculate [IO3]23 for each sample. NOTE: Use xy key on your calculator. Show a sample calculation of [H+]for tube \#2. ( 1 mark) 2. Plot a graph of [IO3]total2vv. [H+]and draw the best-fit straight line through the points. Refer to I\&A "Graphing Requirements". Attach the labeled graph to your report. (3 marks) 3. Determine the slope and y-intercept when [H]=0. Show calculations on your graph. ( 2 marks) 4. Calculate the Ksp of Ca(IO3)2 and the K2 of HIO3. Refer to equations [8] through [10]. Show calculations below. ( 2 marks) 5. Calculate the % error in your values of Ksp and Ka compared to the following values obtained by previous lab sections: Ksp=3.3105;Ka=0.48 at 25C (1 mark) 6. Explain how the following determinate errors would affect your calculated value for Ksp. a) The calcium iodate solutions were not completely saturated. ( 1 mark) b) Some solid Ca(IO3)2 was present in the aliquot of filtrate used for analysis. (1 mark) 7. What appears to be the largest source of determinate error in your experimental determination of the Ksp ? Explain. (1 mark)