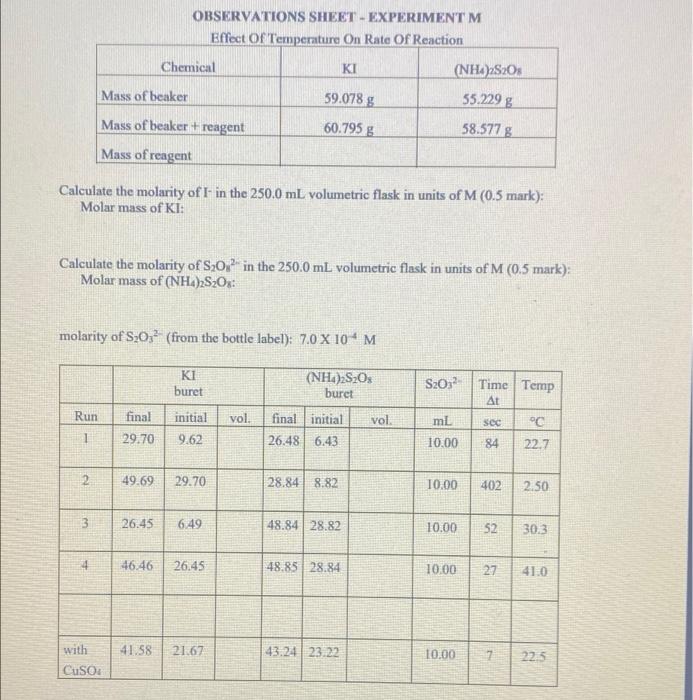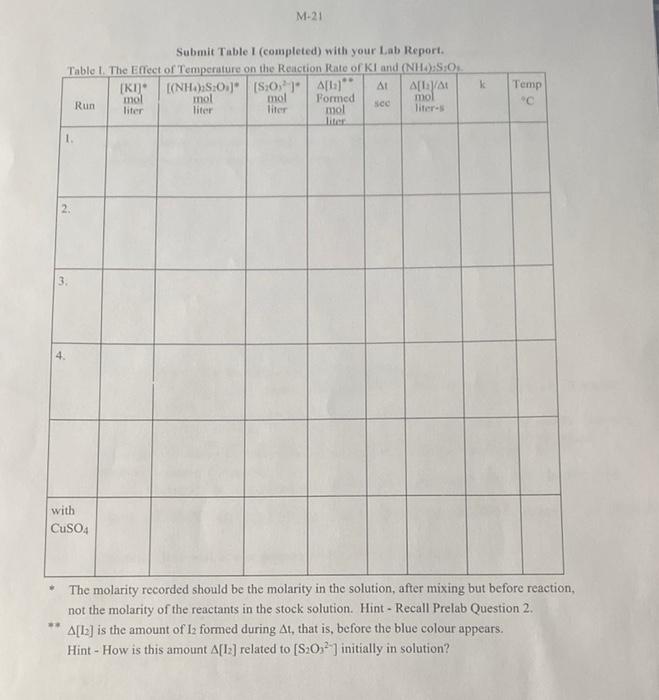
OBSERVATIONS SHEET - EXPERIMENT M Effect of Temperature On Rate Of Reaction Chemical KI (NH4)2S2O3 Mass of beaker 59.078 g 35.229 g 60.795 g 58.577 g Mass of beaker + reagent Mass of reagent Calculate the molarity of I- in the 250.0 mL volumetric flask in units of M (0.5 mark): Molar mass of KI: Calculate the molarity of S20% in the 250.0 mL volumetric flask in units of M (0.5 mark); Molar mass of (NH4)2S2Os: molarity of S20; (from the bottle label): 7,0 X 104 M KI buret (NH4)2S2O3 buret Run vol. vol final 29.70 initial 9.62 final initial 26.48 6.43 S2052 Time Temp At mL sec C 10.00 84 22.7 1 2 49.69 29.70 28.84 8.82 10.00 402 2.50 3 3 26.45 6:49 48.84 28.82 10.00 52 30.3 4 46.46 26.45 48.85 28.84 10.00 27 41.0 41.58 21.67 43.24 23:22 with CuSO4 10.00 7 22.5 - Show your work answers without supporting work will get zero marks, Give units and use the correct number of significant figure, 1. Complete Table (page M-21) and include it with this Postlab assignment (2 marks) 2. Show your calculations for Run I below. Include units. (1 mark) 3 Complete the following table relating the temperature to reaction rate. (1 mark) Temperature k (from Table 1) Ink T(K) T("C) T(K) () Use the graph paper is given at the back of this Lab Manual. Plot Ink against 1/T with Tin degrees K on a suitable scale and submit it with this Postlab assignment (see Guidelines on Graphing, p. 39 and 40 of this Manual). Alternatibely an Excel plot may be used provided the plot is expanded to the same size of the graphing paper. Draw a straight line best-fit curve through the noncatalyzed data points. (2 marks) (b) Show on your graph your calculation (in ink) of (1) the slope of the line (1 mark) (ii) E. (1 mark) (Questions continue on the next page) M-21 Submit Table I (completed) with your Lab Report. Table 1. The Effect of Temperature on the Reaction Rate of kland (NHOS:O [R1]* | [(NH4)2SO4]* | [S20]* | A[-]"" A[/A mol mol mol Run Formed mol liter liter liter sed mol literas liter 1. Temp C 3 with CuSO4 The molarity recorded should be the molarity in the solution, after mixing but before reaction, not the molarity of the reactants in the stock solution. Hint - Recall Prelab Question 2. A[12] is the amount of 12 formed during At, that is, before the blue colour appears. Hint - How is this amount A[12] related to [S202 ) initially in solution