Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ochem please help double check my answers and help with blank answers. thank you Centauri 3. (1 point) If a compound has a large AT
ochem 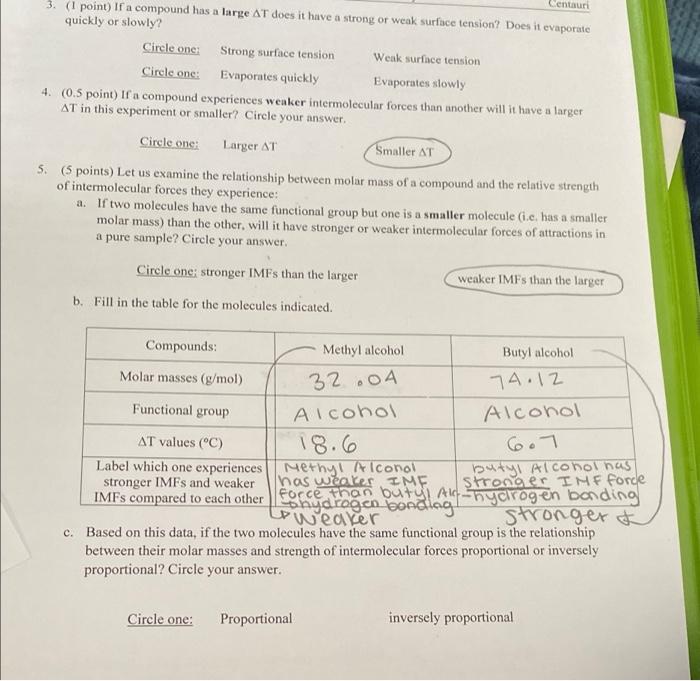
Centauri 3. (1 point) If a compound has a large AT does it have a strong or weak surface tension? Does it evaporate quickly or slowly? Circle one Strong surface tension Weak surface tension Circle ons: Evaporates quickly Evaporates slowly 4. (0.5 point) If a compound experiences weaker intermolecular forces than another will it have a larger AT in this experiment or smaller? Circle your answer. Circle one: Larger AT Smaller AT 5. (5 points) Let us examine the relationship between molar mass of a compound and the relative strength of intermolecular forces they experience: a. If two molecules have the same functional group but one is a smaller molecule (i.c. has a smaller molar mass) than the other, will it have stronger or weaker intermolecular forces of attractions in a pure sample? Circle your answer. Circle one stronger IMFs than the larger weaker IMFs than the larger b. Fill in the table for the molecules indicated. Functional group Compounds: Methyl alcohol Butyl alcohol Molar masses (g/mol) 32.04 74.12 Alcohol Alcohol AT values (C) 18.6 6.7 Label which one experiences Methy! Alconal butyl Alcohol has stronger IMFs and weaker has weaker IME Stronger IMF forde IMFs compared to each other forecame buteladanyarogen bonding nya stronger of c. Based on this data, if the two molecules have the same functional group is the relationship between their molar masses and strength of intermolecular forces proportional or inversely proportional? Circle your answer. Circle one: Proportional inversely proportional please help double check my answers and help with blank answers.
thank you

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


