Question
? If 4.0 g of acetanilide are mixed with 1.5 mL of 16M nitric acid and excess sulfuric acid, then calculate the theoretical yield of
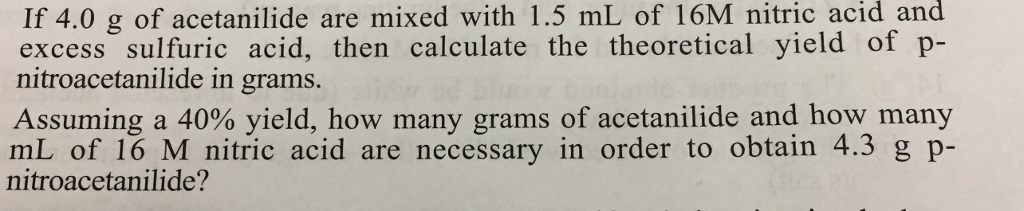
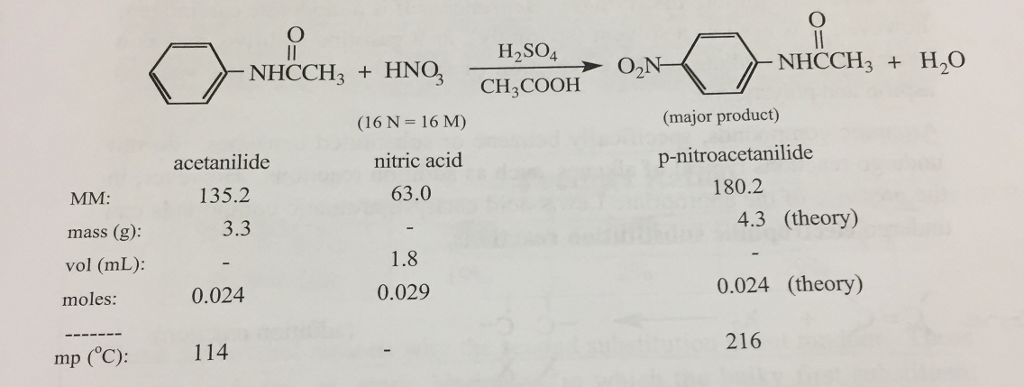
If 4.0 g of acetanilide are mixed with 1.5 mL of 16M nitric acid and excess sulfuric acid, then calculate the theoretical yield of p- nitroacetanilide in grams. Assuming a 40% yield, how many grams of acetanilide and how many mL of 16 M nitric acid are necessary in order to obtain 4.3 g p- nitroacetanilide? MM: mass (g): vol (ml): moles: mp (C): acetanilide 135.2 3.3 0.024 NHCCH3 + HNO3 114 (16 N = 16 M) nitric acid 63.0 - 1.8 0.029 HSO4 CH3COOH NHCCH3 + H,O (major product) p-nitroacetanilide 180.2 4.3 (theory) 0.024 (theory) 216
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Ans Theor...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



