Answered step by step
Verified Expert Solution
Question
1 Approved Answer
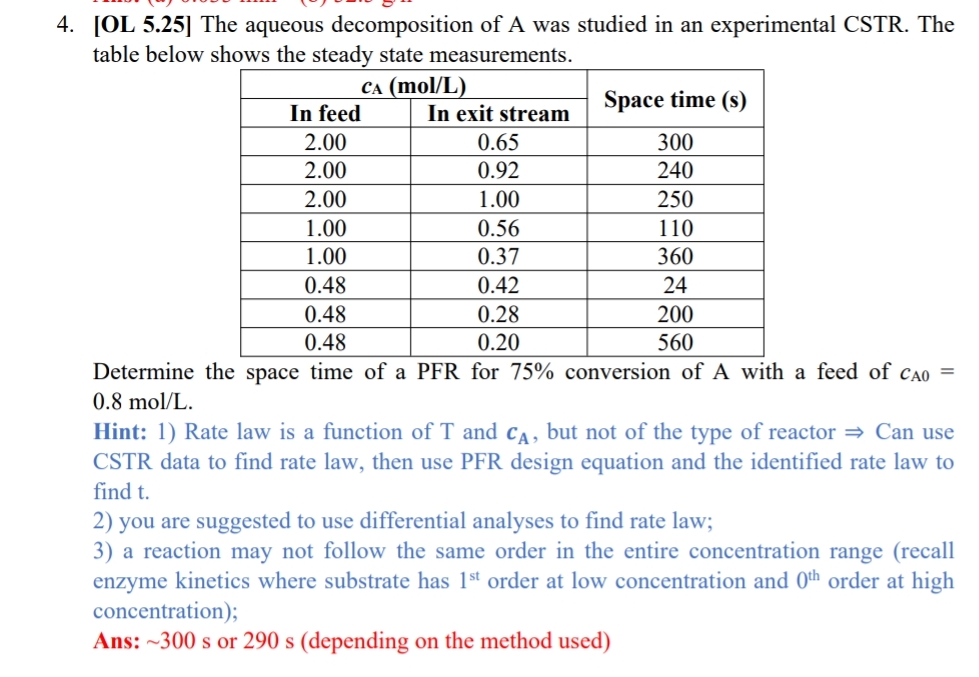
[ OL 5 . 2 5 ] The aqueous decomposition of A was studied in an experimental CSTR . The table below shows the steady
OL The aqueous decomposition of A was studied in an experimental CSTR The table below shows the steady state measurements.
tableSpace time sIn feed,In exit stream
Determine the space time of a PFR for conversion of A with a feed of
Hint: Rate law is a function of and but not of the type of reactor Can use CSTR data to find rate law, then use PFR design equation and the identified rate law to find
you are suggested to use differential analyses to find rate law;
a reaction may not follow the same order in the entire concentration range recall enzyme kinetics where substrate has order at low concentration and order at high concentration;
Ans: or depending on the method used
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


