Answered step by step
Verified Expert Solution
Question
1 Approved Answer
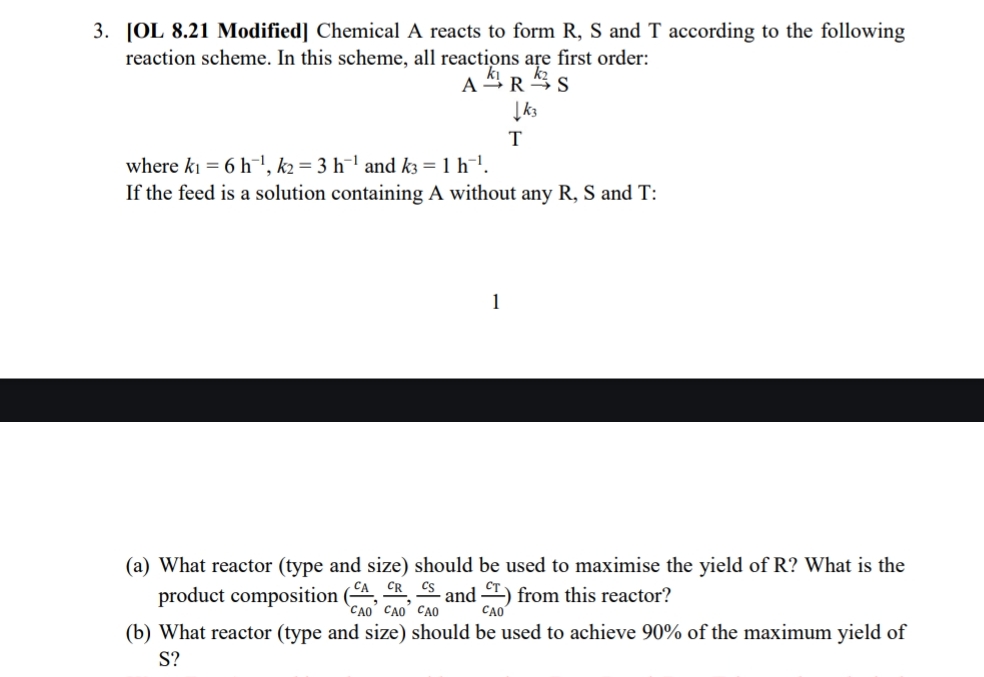
[ OL 8 . 2 1 Modified ] Chemical A reacts to form R , S and T according to the following reaction scheme. In
OL Modified Chemical A reacts to form R S and T according to the following reaction scheme. In this scheme, all reactions are first order:
where and
If the feed is a solution containing A without any R S and T:
a What reactor type and size should be used to maximise the yield of R What is the product composition and : from this reactor?
b What reactor type and size should be used to achieve of the maximum yield of S
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


