Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Old MathJax webview combustion engineering please answer it all and guarantee a thumbs up that's all. no pressure and temp included in the question Direction:
Old MathJax webview
combustion engineering
please answer it all and guarantee a thumbs up
that's all. no pressure and temp included in the question
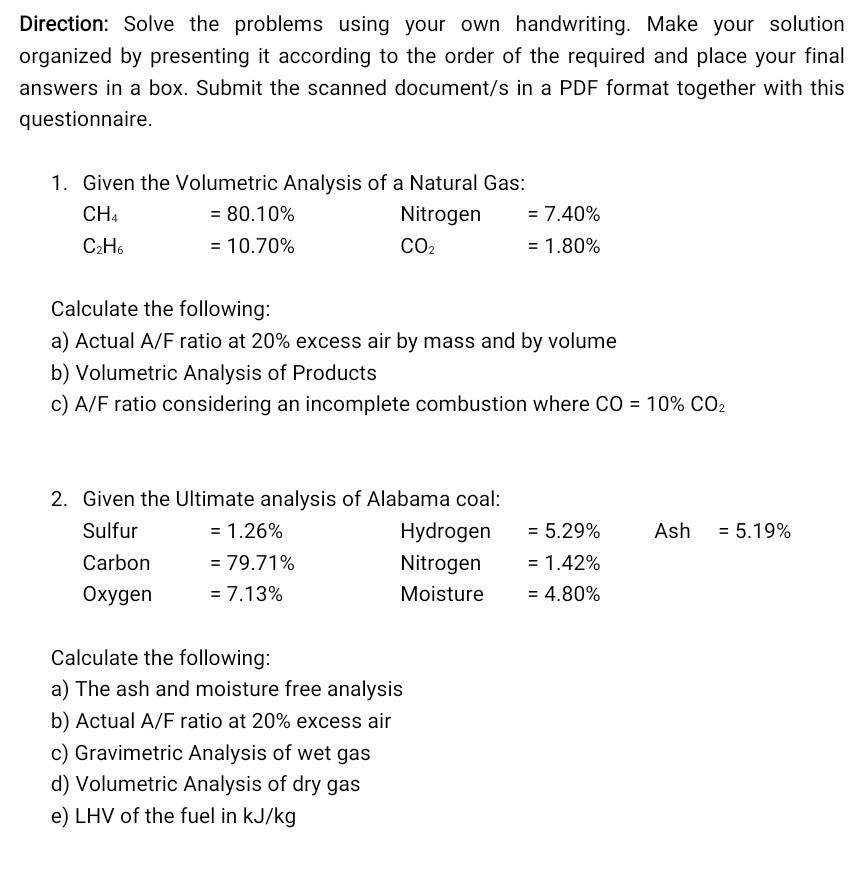
Direction: Solve the problems using your own handwriting. Make your solution organized by presenting it according to the order of the required and place your final answers in a box. Submit the scanned document/s in a PDF format together with this questionnaire. 1. Given the Volumetric Analysis of a Natural Gas: CH4 = 80.10% Nitrogen = 7.40% C2H6 = 10.70% CO2 = 1.80% Calculate the following: a) Actual A/F ratio at 20% excess air by mass and by volume b) Volumetric Analysis of Products c) A/F ratio considering an incomplete combustion where CO = 10% CO2 Ash = 5.19% 2. Given the Ultimate analysis of Alabama coal: Sulfur = 1.26% Hydrogen Carbon = 79.71% Nitrogen Oxygen = 7.13% Moisture = 5.29% = 1.42% = 4.80% Calculate the following: a) The ash and moisture free analysis b) Actual A/F ratio at 20% excess air c) Gravimetric Analysis of wet gas d) Volumetric Analysis of dry gas e) LHV of the fuel in kJ/kgStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


