Answered step by step
Verified Expert Solution
Question
1 Approved Answer
on jupyter notebook Plank's radiation law says that the intensity of radiation per unit area and per unit wavelength i from a black body at
on jupyter notebook
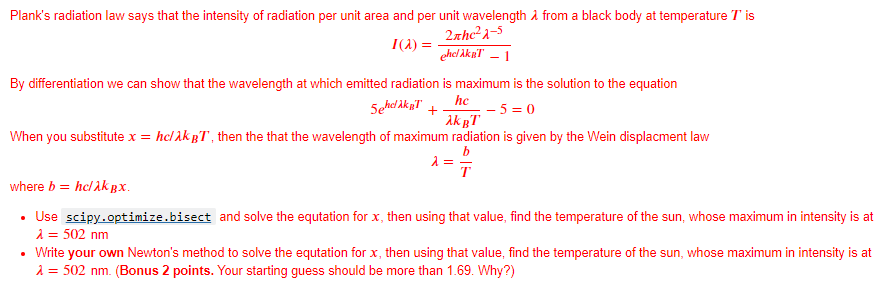
Plank's radiation law says that the intensity of radiation per unit area and per unit wavelength i from a black body at temperature T is TA) = 2xhc21-5 " ghe/ikpT - 1 By differentiation we can show that the wavelength at which emitted radiation is maximum is the solution to the equation :-5 = 0 When you substitute x = hcik T, then the that the wavelength of maximum radiation is given by the Wein displacment law Sehelaka! + TRT where b= hc/ik sx. Use scipy.optimize.bisect and solve the equtation for x, then using that value, find the temperature of the sun, whose maximum in intensity is at i= 502 nm Write your own Newton's method to solve the equtation for x, then using that value, find the temperature of the sun, whose maximum in intensity is at 1 = 502 nm. (Bonus 2 points. Your starting guess should be more than 1.69. Why?) Plank's radiation law says that the intensity of radiation per unit area and per unit wavelength i from a black body at temperature T is TA) = 2xhc21-5 " ghe/ikpT - 1 By differentiation we can show that the wavelength at which emitted radiation is maximum is the solution to the equation :-5 = 0 When you substitute x = hcik T, then the that the wavelength of maximum radiation is given by the Wein displacment law Sehelaka! + TRT where b= hc/ik sx. Use scipy.optimize.bisect and solve the equtation for x, then using that value, find the temperature of the sun, whose maximum in intensity is at i= 502 nm Write your own Newton's method to solve the equtation for x, then using that value, find the temperature of the sun, whose maximum in intensity is at 1 = 502 nm. (Bonus 2 points. Your starting guess should be more than 1.69. Why?)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


