Question: The student performs a second titration using the 0.10 M NAOH(aq) solution again as the titrant, but this time with a 20. mL sample
![]()
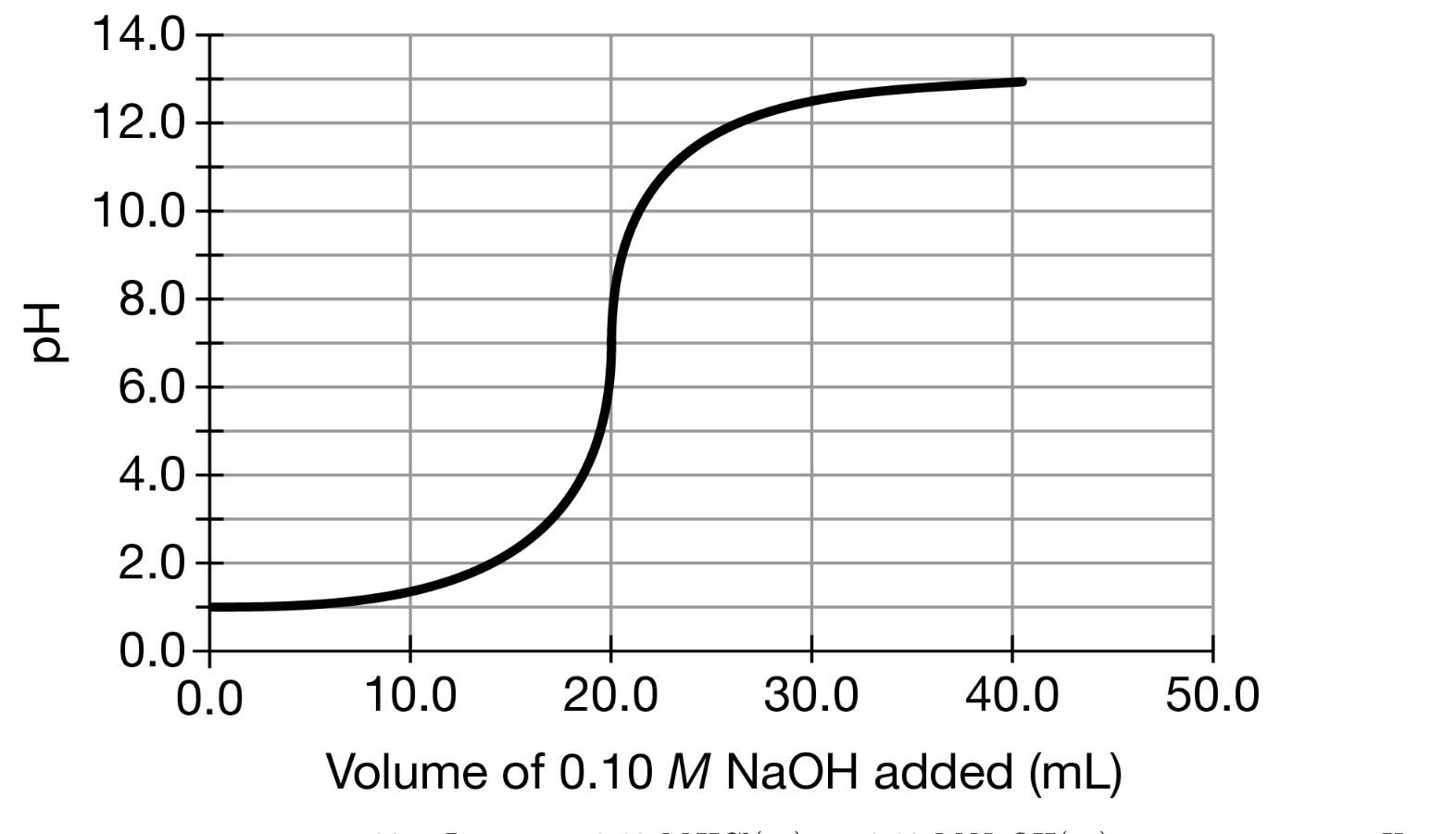
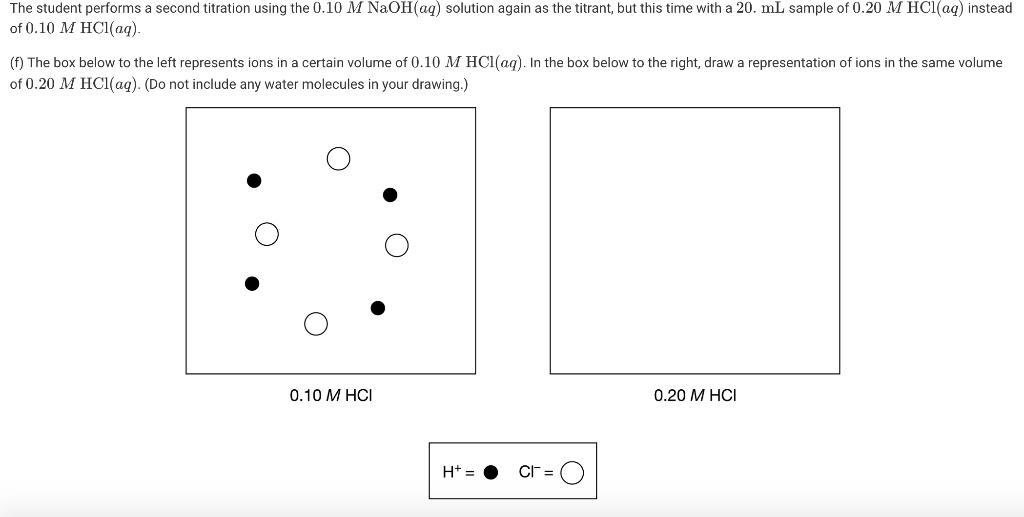
The student performs a second titration using the 0.10 M NAOH(aq) solution again as the titrant, but this time with a 20. mL sample of 0.20 M HCl(aq) instead of 0.10 M HC1(aq). (f) The box below to the left represents ions in a certain volume of 0.10 M HCl(aq). In the box below to the right, draw a representation of ions in the same volume of 0.20 M HC1(aq). (Do not include any water molecules in your drawing.) 0.10 M HCI 0.20 M HCI H+ = CF = (h) The student made observations related to the contents of the Erlenmeyer flask during the titration. Identify an observation that could have led the student to conclude that a chemical change took place during the titration. 14.0- 12.0- 10.0 8.0 6.0 4.0 2.0 0.0+ 0.0 10.0 20.0 30.0 40.0 50.0 Volume of 0.10 M NaOH added (mL)
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


