Answered step by step
Verified Expert Solution
Question
1 Approved Answer
one bonding pairs four two singles lone valence six less lower Complete and correctly sequence the steps for drawing Lewis structures. -Drag the text
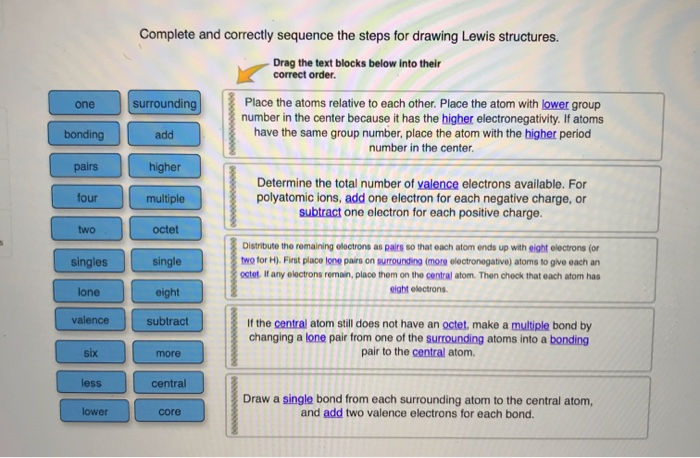
one bonding pairs four two singles lone valence six less lower Complete and correctly sequence the steps for drawing Lewis structures. -Drag the text blocks below into their correct order. surrounding add higher multiple octet single eight subtract more central core Place the atoms relative to each other. Place the atom with lower group number in the center because it has the higher electronegativity. If atoms have the same group number, place the atom with the higher period number in the center. Determine the total number of valence electrons available. For polyatomic ions, add one electron for each negative charge, or subtract one electron for each positive charge. Distribute the remaining electrons as pairs so that each atom ends up with eight electrons (or two for H). First place lone pairs on surrounding (more electronegative) atoms to give each an octet. If any electrons remain, place them on the central atom. Then check that each atom has eight electrons. If the central atom still does not have an octet, make a multiple bond by changing a lone pair from one of the surrounding atoms into a bonding pair to the central atom. Draw a single bond from each surrounding atom to the central atom, and add two valence electrons for each bond.
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Step4 Count Valence elections in the molecule or ion Do this by adding the Periodic each at...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


