Question
One mole of propane is being burned completely and adiabatically with five moles of oxygen via the reaction: CHs(g) +50(g) 3CO(g) + 4HO(g) The
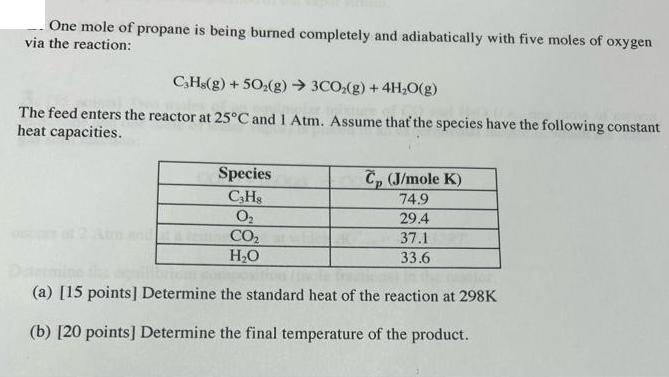
One mole of propane is being burned completely and adiabatically with five moles of oxygen via the reaction: CHs(g) +50(g) 3CO(g) + 4HO(g) The feed enters the reactor at 25C and 1 Atm. Assume that the species have the following constant heat capacities. Species CH8 0 CO HO C (J/mole K) 74.9 29.4 37.1 33.6 (a) [15 points] Determine the standard heat of the reaction at 298K (b) [20 points] Determine the final temperature of the product.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer to Propane Combustion Problem Part a Standard Heat of Reaction at 298 K 15 points Standard Enthalpy of Formation Use reference data to find the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



