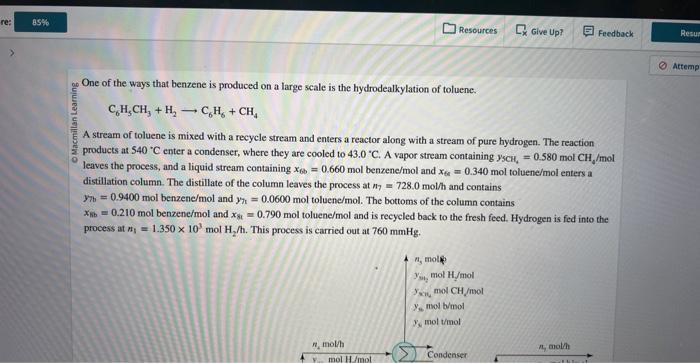
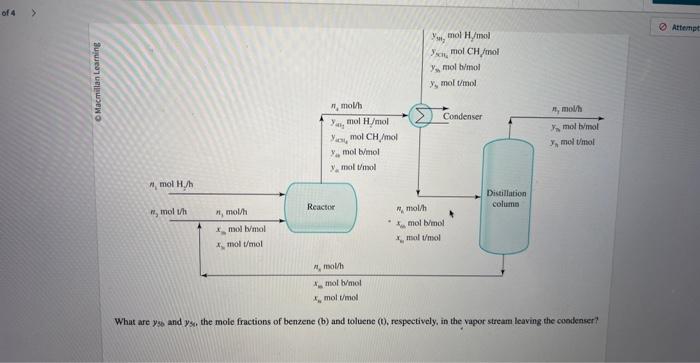
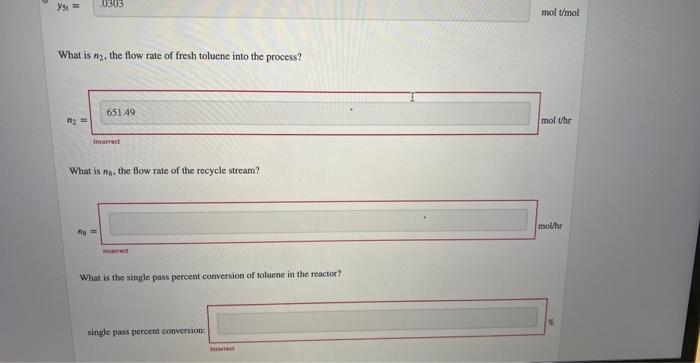
One of the ways that benzene is produced on a large scale is the hydrodealkylation of toluene. C6H5CH3+H2C6H6+CH4 A stream of toluene is mixed with a recycle stream and enters a reactor along with a stream of pure hydrogen. The reaction products at 540C enter a condenser, where they are cooled to 43.0C. A vapor stream coataining y5CH4=0.580molCH/mol leaves the process, and a liquid stream containing x6b=0.660mol benzene/mol and x6b=0.340mol toluene/mol enters a distillation column. The distillate of the column leaves the process at n7=728.0mol/h and contains y7t=0.9400mol benzene /mol and y7r=0.0600mol toluene/mol. The bottoms of the column contains xb=0.210mol benzene/mol and x8t=0.790mol toluene/mol and is recycled back to the fresh feed. Hydrogen is fed into the process at n1=1.350103molHH2/h. This process is carried out at 760mmHg. What are y56 and y50, the mole fractions of benzene (b) and toluene ( 0 , respectively, in the vapor stream leaving the coodenser? What is n2, the flow rate of fresh toluene into the process? interect What is n8, the flow rate of the recycle stream? micerect What is the single pass percent conversion of toluene in the reactor? single pass percent conversion One of the ways that benzene is produced on a large scale is the hydrodealkylation of toluene. C6H5CH3+H2C6H6+CH4 A stream of toluene is mixed with a recycle stream and enters a reactor along with a stream of pure hydrogen. The reaction products at 540C enter a condenser, where they are cooled to 43.0C. A vapor stream coataining y5CH4=0.580molCH/mol leaves the process, and a liquid stream containing x6b=0.660mol benzene/mol and x6b=0.340mol toluene/mol enters a distillation column. The distillate of the column leaves the process at n7=728.0mol/h and contains y7t=0.9400mol benzene /mol and y7r=0.0600mol toluene/mol. The bottoms of the column contains xb=0.210mol benzene/mol and x8t=0.790mol toluene/mol and is recycled back to the fresh feed. Hydrogen is fed into the process at n1=1.350103molHH2/h. This process is carried out at 760mmHg. What are y56 and y50, the mole fractions of benzene (b) and toluene ( 0 , respectively, in the vapor stream leaving the coodenser? What is n2, the flow rate of fresh toluene into the process? interect What is n8, the flow rate of the recycle stream? micerect What is the single pass percent conversion of toluene in the reactor? single pass percent conversion