Answered step by step
Verified Expert Solution
Question
1 Approved Answer
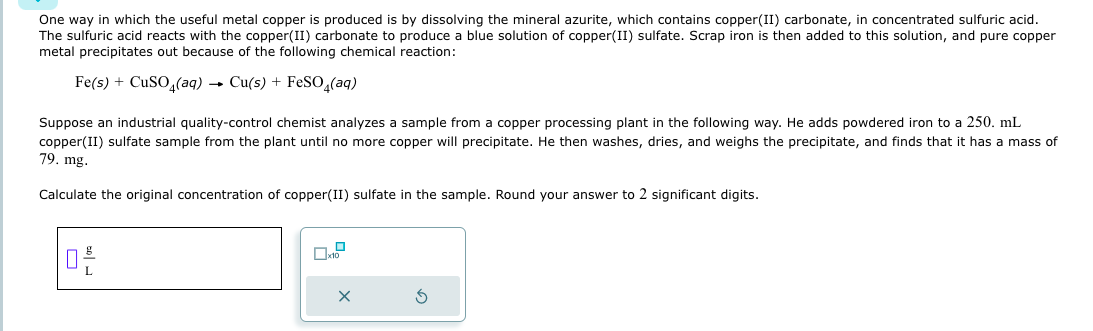
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper ( II ) carbonate, in concentrated
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copperII carbonate, in concentrated sulfuric acid.
The sulfuric acid reacts with the copperII carbonate to produce a blue solution of copperII sulfate. Scrap iron is then added to this solution, and pure copper
metal precipitates out because of the following chemical reaction:
Suppose an industrial qualitycontrol chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a
copperII sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of
Calculate the original concentration of copperII sulfate in the sample. Round your answer to significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


