Answered step by step
Verified Expert Solution
Question
1 Approved Answer
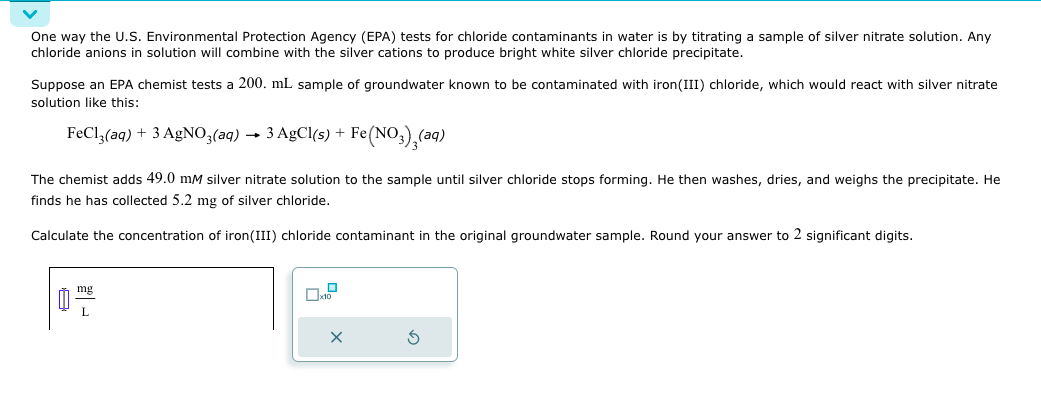
One way the U . S . Environmental Protection Agency ( EPA ) tests for chloride contaminants in water is by titrating a sample of
One way the US Environmental Protection Agency EPA tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any
chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate.
Suppose an EPA chemist tests a mL sample of groundwater known to be contaminated with ironIII chloride, which would react with silver nitrate
solution like this:
AgCl
The chemist adds silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He
finds he has collected of silver chloride.
Calculate the concentration of ironIII chloride contaminant in the original groundwater sample. Round your answer to significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


