Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One way to make vinegar ( not the preferred way) is to prepare a solution of acetic acid, the sole acid component of vinegar, at
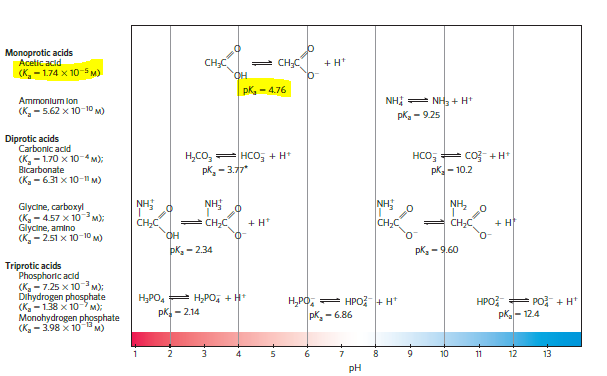
One way to make vinegar (not the preferred way) is to prepare a solution of acetic acid, the sole acid component of vinegar, at the proper pH (see Fig. 2-15) and add appropriate flavoring agents. Acetic acid (Mr 60) is a liquid at 25C, with a density of 1.049 g/mL. Calculate the volume that must be added to distilled water to make 1 L of simulated vinegar(see Fig. 2-16).
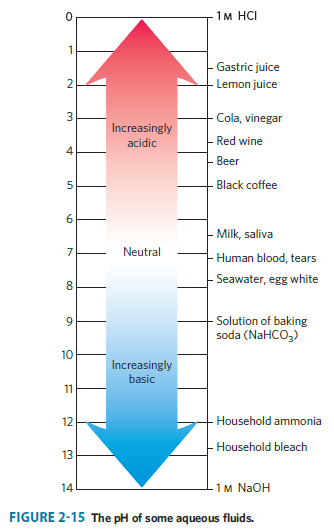
1 2 3 4 5 6 7 8 9 10 11 12 13 14 Increasingly acidic Neutral Increasingly basic 1 M HCI Gastric juice Lemon juice Cola, vinegar -Red wine Beer -Black coffee -Milk, saliva -Human blood, tears -Seawater, egg white Solution of baking soda (NaHCO3) -Household ammonia -Household bleach 1M NaOH FIGURE 2-15 The pH of some aqueous fluids.
Step by Step Solution
★★★★★
3.37 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
To prepare 1 L of simulated vinegar we need to calculate the volume of acetic acid that must be adde...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


