Answered step by step
Verified Expert Solution
Question
1 Approved Answer
only 2 and 4 are wrong N-bromosuccinimide (NBS) controls the rates of competing reactions by minimizing the concentration of Brz produced in the reaction. Draw
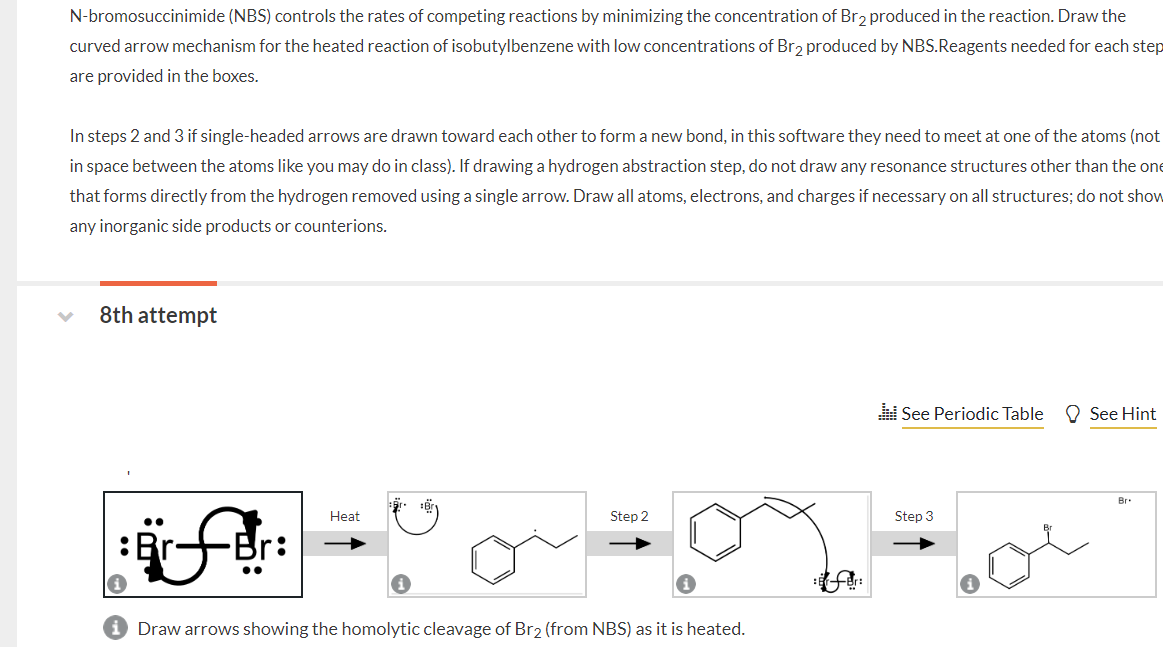
only 2 and 4 are wrong
N-bromosuccinimide (NBS) controls the rates of competing reactions by minimizing the concentration of Brz produced in the reaction. Draw the curved arrow mechanism for the heated reaction of isobutylbenzene with low concentrations of Bra produced by NBS. Reagents needed for each step are provided in the boxes. In steps 2 and 3 if single-headed arrows are drawn toward each other to form a new bond, in this software they need to meet at one of the atoms (not in space between the atoms like you may do in class). If drawing a hydrogen abstraction step, do not draw any resonance structures other than the one that forms directly from the hydrogen removed using a single arrow. Draw all atoms, electrons, and charges if necessary on all structures; do not show any inorganic side products or counterions. 8th attempt See Periodic Table See Hint Br Heat Step 2 Step 3 for Draw arrows showing the homolytic cleavage of Br2 (from NBS) as it is heatedStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


