Answered step by step
Verified Expert Solution
Question
1 Approved Answer
only d 92-7 The adiabatic exothermic irreversible gas-phase reaction 2A+B2C is to be carried out in a flow reactor for an equimolar feed of A
only d 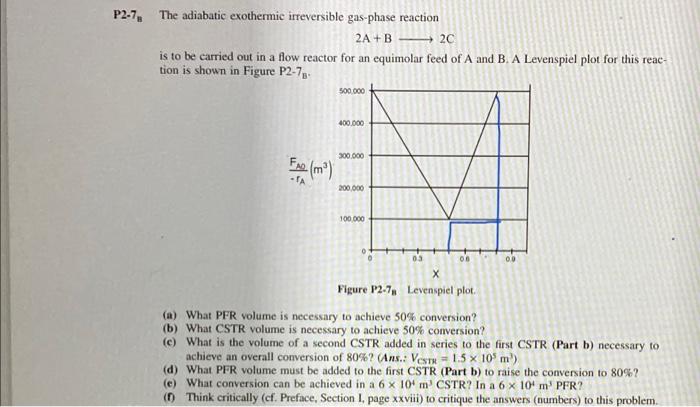
92-7 The adiabatic exothermic irreversible gas-phase reaction 2A+B2C is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in Figure P27H. (a) What PFR volume is necessary to achieve 50% conversion? (b) What CSTR volume is necessary to achieve 50% conversion? (c) What is the volume of a second CSTR added in series to the first CSTR (Part b) necessary to achieve an overall conversion of 80% ? (Ans.: VCrx=1.5105m3 ) (d) What PFR volume must be added to the first CSTR (Part b) to raise the conversion to 80% ? (c) What conversion can be achieved in a 6104m3 CSTR? In a 6104m3 PFR? (f) Think critically (cf. Preface, Section I, page xxv viii) to critique the answers (numbers) to this 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


