Answered step by step
Verified Expert Solution
Question
1 Approved Answer
only need the second one thanks! If a pork roast must absorb 1.7 x 109 kJ to fully cook, and if only 10 % of

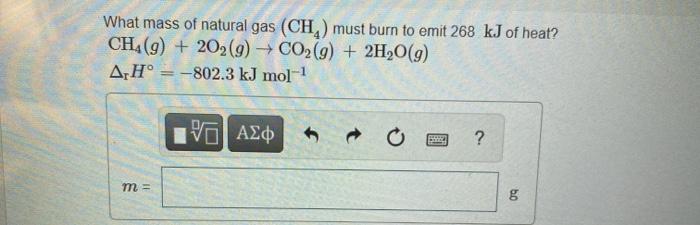
only need the second one thanks!
If a pork roast must absorb 1.7 x 109 kJ to fully cook, and if only 10 % of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast? Express your answer using two significant figures. ? MCO, 8 What mass of natural gas (CH) must burn to emit 268 kJ of heat? CH4(9) + 202(9) + CO2(g) + 2H2O(9) ArH = -802.3 kJ mol-1 VALO BTC ? m = bo Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


