Question
Organic micropollutants (MP), such as well-known Bisphenol A, are mainly anthropogenic molecules found ubiquitously in our everyday products and materials (plastics, antibacterial products, detergents, medication).
Organic micropollutants (MP), such as well-known Bisphenol A, are mainly anthropogenic molecules found ubiquitously in our everyday products and materials (plastics, antibacterial products, detergents, medication). MP are present in urban or industrial wastewater at low concentrations (g/l or ng/l). However, the main issue of these molecules is that they already possess an environmental toxicity at these low concentrations (e.g. hormonal activity disturbing the hormonal balance of aquatic organisms).
The wastewater treatment plants classical processes do not provide any sufficient removal of MP due to their lack of specificity. MP concentrations being low, elimination yields remain low. This is why, nowadays, researchers are investigating new technologies of MP degradation in wastewaters. One of the possible techniques is the use of ligninolytic enzymes, as laccases from the White Rot fungi. These enzymes show a great affinity for the phenolic structure, characteristic of most of MP. The use of a packed bed of immobilized laccases in a column presents promising results. The supports for the immobilization can be of various kinds (silica beads, silica powder, alginates...)
Problem
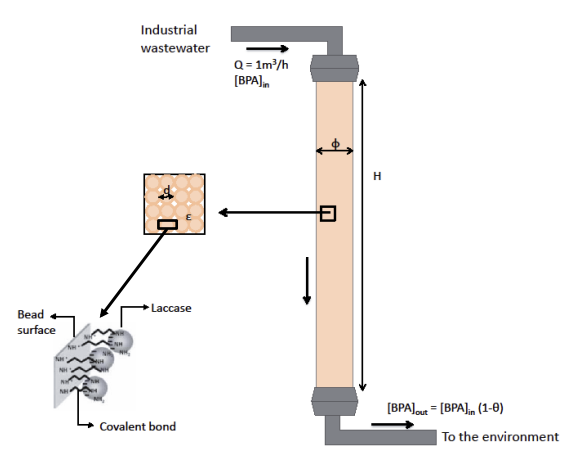
A plastics manufacturing plant discharges Q = 1 m3 /h of wastewater containing a harmful concentration of BPA. Before sending the wastewater to the aquatic environment, the concentration of BPA has to be reduced by 90% (conversion =0.9). A stainless steel column filled with laccases immobilized on silica beads is used for this purpose (see Figure below). The column can be considered as a plug flow tubular reactor (PFTR). Two steps in series can limit the rate of the degradation process: the diffusion of the BPA through the diffusion boundary layer in the liquid around the beads and the BPA degradation reaction by the immobilized laccases. Based on the diffusion theory, the thickness of the boundary layer around the beads, xl, can be obtained from the following expression:
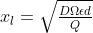
where D is the diffusion coefficient of the BPA in water, is the area of the flow cross section of the column, is the porosity of the stack composed by the silica beads, and d is the diameter of the silica beads.
A stationary regime is assumed, meaning that there is no accumulation of the MP on the surface of the beads: the rate of the chemical degradation by the laccases is equal to the rate of the diffusion through the boundary layer.
At the surface of the beads, the rate r at which the BPA is degraded (expressed in moles per second and per unit surface of the beads) can be expressed by the following equation:
r = k Cs,
where k is a kinetic constant (m/s) and Cs is the BPA concentration at the surface of the beads. Such an equation can be derived from the classical Michaelis-Menten equation, assuming that the BPA concentration is widely lower than the Michaelis constant characteristic of the enzyme-MP reaction.
Questions
1. In order to achieve the desired objective, what should be the dimensions of the column (, H, ), knowing that we choose to work with H = 10 , where is the diameter of the column?
2. Using the Kozeny-Karman model to express the permeability of the stack composed by the silica beads, calculate the loss of driving pressure (p) across the column? Is the water able to flow through the column under its own weight? If not, which additional pressure difference should be imposed with a pump connected at the inlet of the column?
3. Which of the two steps in series limit the rate of the degradation process?
Dimensions
- Diameter of the beads: d = 0,4 mm
- Porosity of the stack of beads: = 0,4
- Diffusion coefficient of BPA in the wastewater: D = 10-10 m2 /s
- Kinetic constant (determined by lab experiments): k= 6 10-7 m/s
- Viscosity of the wastewater: = 10-3 kg/(m.s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


