Answered step by step
Verified Expert Solution
Question
1 Approved Answer
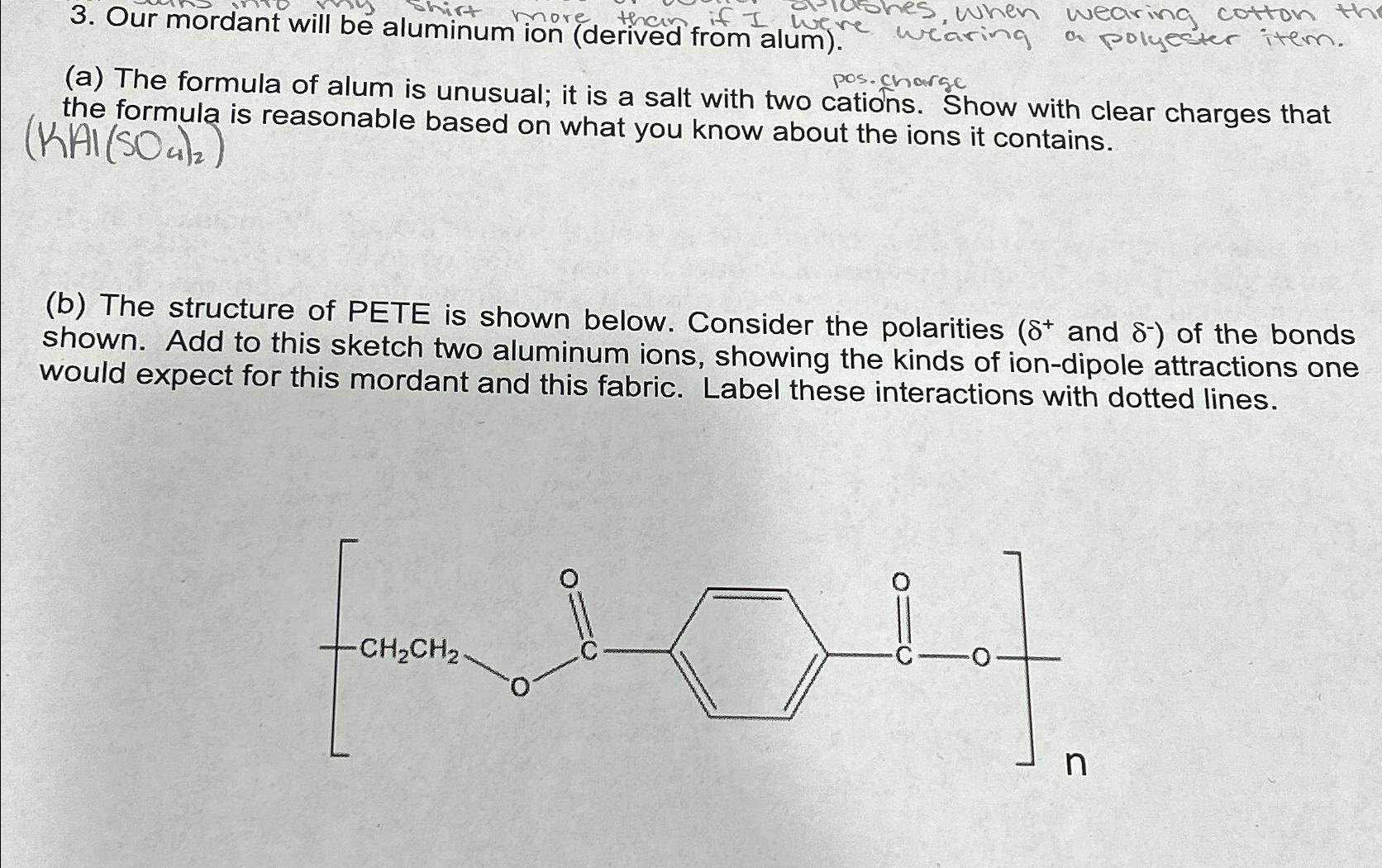
Our mordant will be aluminum ion ( derived from alum ) . pos. charge ( a ) The formula of alum is unusual; it is
Our mordant will be aluminum ion derived from alum
pos. charge
a The formula of alum is unusual; it is a salt with two cations. Show with clear charges that the formula is reasonable based on what you know about the ions it contains.
b The structure of PETE is shown below. Consider the polarities and :of the bonds shown. Add to this sketch two aluminum ions, showing the kinds of iondipole attractions one would expect for this mordant and this fabric. Label these interactions with dotted lines.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


