Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Oxalic acid, HOOC-COOH, is the simplest acid that has two carboxylic acid groups. Write an equation and show the structure of a polyester that might
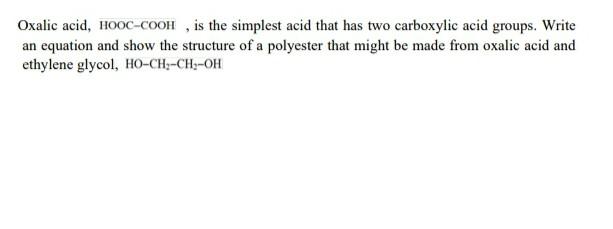
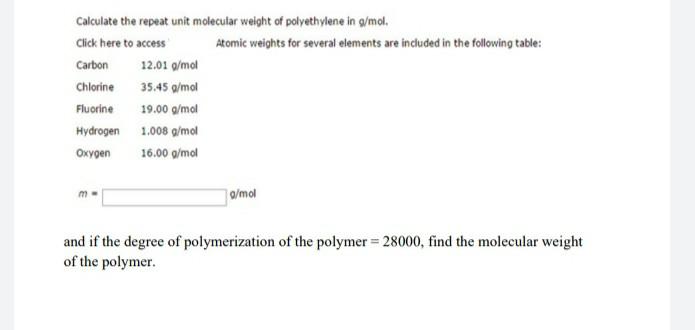


Oxalic acid, HOOC-COOH, is the simplest acid that has two carboxylic acid groups. Write an equation and show the structure of a polyester that might be made from oxalic acid and ethylene glycol, HO-CH2-CH2-OH Calculate the repeat unit molecular weight of polyethylene in g/mol. Click here to access Atomic weights for several elements are included in the following table: Carbon 12.01 g/mol Chlorine 35.45 g/mol Fluorine 19.00 g/mol Hydrogen 1.008 g/mol Oxygen 16.00 g/mol g/mol and if the degree of polymerization of the polymer = 28000, find the molecular weight of the polymer. Suggest the ionic polymerization mechanism of the following monomers 1- vinyl chloride CH=CHCI vinyl chloride 2. CH,C = CH, 1 CH, 2 Methyl-propene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


